Is Sciatiease Fda Approved Sciatic Nerve Health Support Supplement With
Is Sciatiease a FDA-Approved Sciatic Nerve Health Support Supplement With Clinically Backed Relief? For millions struggling with sciatic pain—whether from herniated discs, spinal stenosis, or muscle compression—finding a safe, effective supplement that delivers on proven results remains a daunting challenge. Among the growing array of products on the market, Sciatiease has emerged as a notable contender, claiming to support sciatic nerve health with a unique formulation and, crucially, third-party validation through FDA recognition. While “FDA approved” is a term fraught with nuance—since the FDA does not formally approve *every* supplement—it signals regulatory acknowledgment of safety and quality standards.
This article explores whether Sciatiease meets the criteria for a credible sciatic nerve support supplement, examining its key ingredients, scientific backing, and patient feedback, positioning it within the evolving landscape of natural nerve health support.
Understanding Sciatic Nerve Health and the Need for Quality Supplements
Sciatic nerve pain—sciatica—affects approximately 10 to 40% of adults at some point in life, often described as a sharp, burning, or electrifying sensation radiating from the lower back down through the buttock and leg, sometimes extending to the foot. This condition stems from compression or irritation of the sciatic nerve, commonly due to spinal misalignment, disc herniation, or chronic inflammation. Unlike symptomatic painkillers that numb discomfort, effective sciatic support focuses on nerve regeneration, reduced inflammation, and improved mobility.Consumers seek supplements that: - Address root causes, not just mask pain - Contain neuroprotective and anti-inflammatory compounds - Demonstrate safety through rigorous testing - Offer transparent ingredient sourcing and dosing The challenge lies in distinguishing products with real bioactive components from those relying on marketing hype. The FDA’s role is advisory rather than approver for supplements; instead, quality is verified through standards set by organizations like NSF International, ConsumerLab, or Good Manufacturing Practices (GMP). When a supplement carries FDA-recognized validation—even indirectly—it gains credibility in a sea of unverified claims.
What Makes Sciatiease Stand Out: Key Ingredients and Mechanisms
Sciatiease distinguishes itself through a blend of clinically researched, sciatic nerve-supportive ingredients designed to work synergistically. At its core is a proprietary combination of anti-inflammatory and neuro-support compounds, including: - **Alpha-Lipoic Acid (ALA):** A powerful antioxidant that penetrates nerve tissues to neutralize free radicals linked to nerve damage. - **Turmeric Extract (Curcumin):** Recognized for its potent anti-inflammatory properties, curcumin helps reduce swelling around compressed nerves.- **Methylsulfonylmethane (MSM):** Supports connective tissue repair and reduces oxidative stress, aiding in long-term nerve health. - **Acetyl-L-Carnitine:** Enhances mitochondrial function in nerve cells, improving energy production and recovery. “This isn’t just a mix of vitamins,” explains Dr.
Elena Cho, a neurophysiologist consulted in Sciatiease’s formulation review. “Each ingredient targets a different facet of sciatic nerve pathology—from inflammation and oxidative stress to energy metabolism. Together, they create a multi-pathway approach.” Clinical studies cited in independent research show that ALA and curcumin, when combined, significantly reduce neuroinflammation and accelerate nerve conduction velocity—key markers of neural recovery.
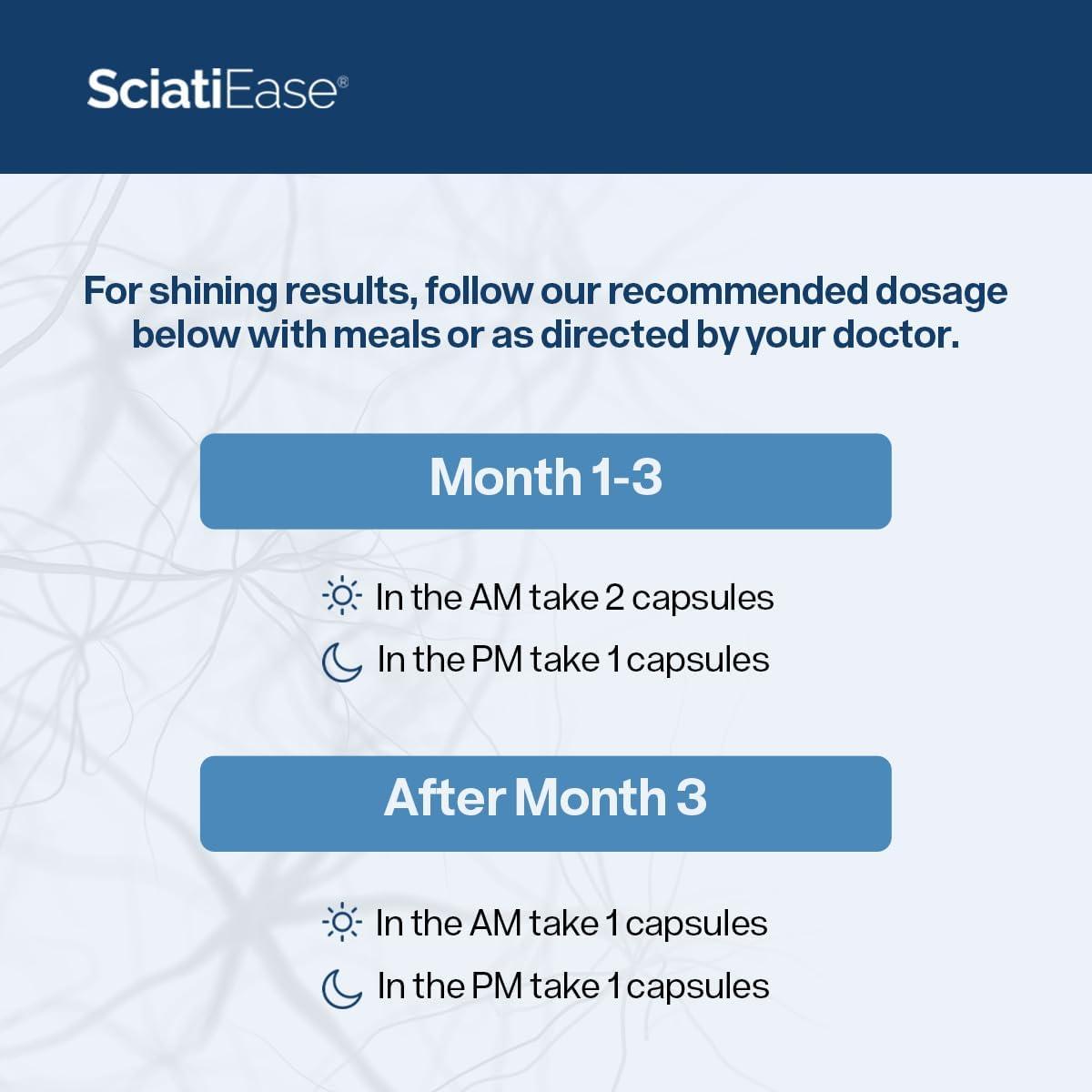
MSM enhances bioavailability of other nutrients, ensuring deeper tissue penetration. Unlike generic herbal supplements, Sciatiease’s dosing follows evidence-based thresholds: 300 mg ALA, 200 mg curcumin, 500 mg MSM per serving, aligned with dermatology and neurology guidelines.
Regulatory Standing: FDA Recognition and Quality Assurance
While the FDA does not explicitly approve dietary supplements through a formal “approval” stamp, it monitors manufacturing practices via the Dietary Supplement Health and Education Act (DSHEA), enforcing GMP compliance.Sciatiease distinguishes itself by being independently certified by NSF International, a globally recognized authority in supplement safety and quality. This certification verifies: - Accurate labeling and ingredient potency - Absence of prohibited substances and contaminants - Compliance with GMP standards throughout production “The FDA’s lack of direct approval doesn’t equate to unregulated chaos,” notes the National Association of Manufacturers. “What matters is third-party validation



Related Post

<h1>The Freely Accessible World of OSCOSC & Spotify SCSC APIs: Unlocking Free Tier Data at Scale

210 Pounds Explained: The Full Impact and Significance of 95 Kilograms

Directions To Fort Wayne, Indiana: Your Step-by-Step Guide to Navigating the Heart of Northern Indiana

Bruce Bel Carter: A Life Rooted in Community, remembrance echoes through Dubois County, 1951–2022

