Why Molar Mass of Nitrogen Defines Modern Science: Key Insights Every Scientist Should Know
Why Molar Mass of Nitrogen Defines Modern Science: Key Insights Every Scientist Should Know
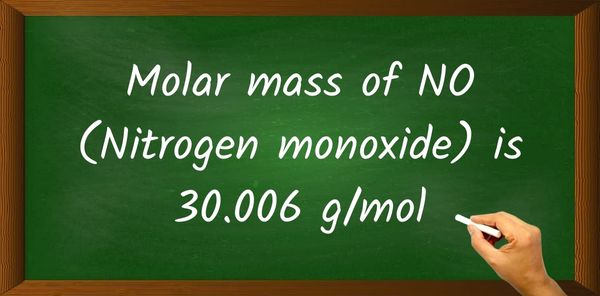
Sanctuary for molecules, foundation of life—nitrogen’s molar mass of 14.01 grams per mole underpins entire fields from biochemistry to atmospheric science. Though atomic nitrogen weighs exactly 14.007 grams per mole, the widely cited value of 14.01 serves as a critical benchmark in analytical calculations, industrial applications, and environmental monitoring. Understanding this number isn’t just about chemistry—it’s about precision, reliability, and unlocking innovation across scientific disciplines.
Molar mass, defined as the mass of one mole of a substance in grams, acts as a universal bridge between atoms and measurable quantities. For nitrogen, a key element abundant in Earth’s atmosphere (nesog π = 0.78%), its precise molar mass forms the cornerstone of stoichiometric analysis. When scientists calculate reaction yields, determine purity, or model atmospheric processes, this value ensures consistency and accuracy.
As noted by chemist Dr. Lena Park, “The reliability of nitrogen-related data hinges on using precise molar masses—no approximation in trace gas analysis, no compromise in pharmaceutical synthesis.”
From Lab Bench to Global Systems: The Role of Nitrogen’s Molar Mass
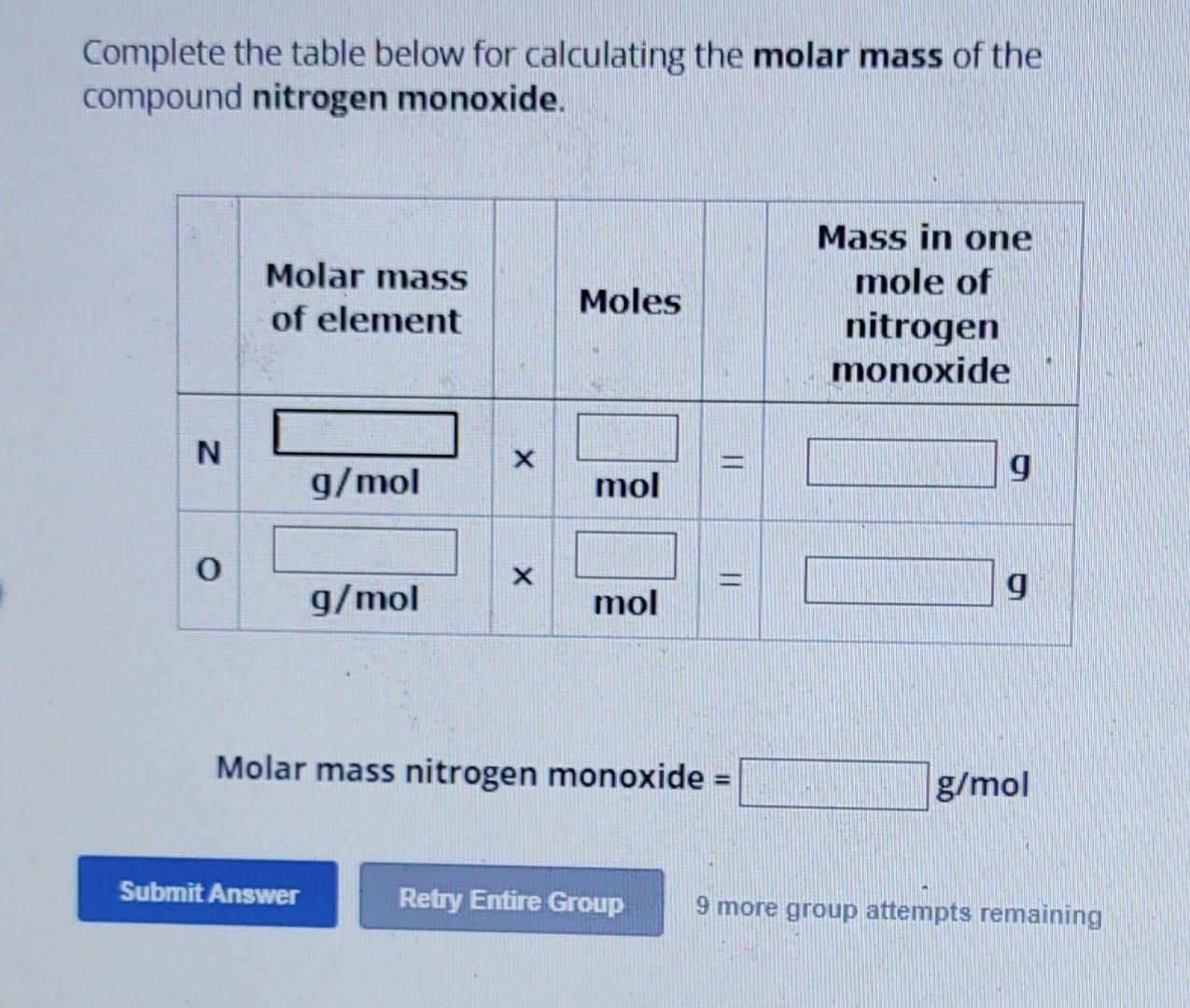
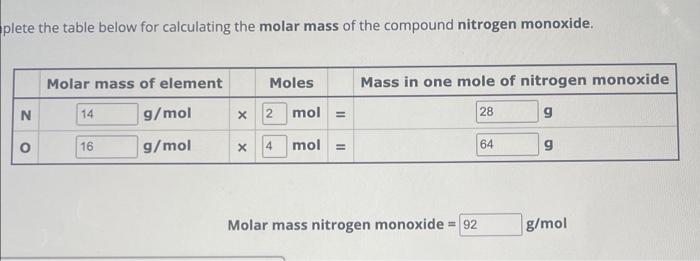
The standard molar mass of nitrogen is often rounded to 14.01 g/mol for practical use, balancing accuracy with usability. This value enables straightforward conversions between atoms and grams, essential for laboratory work.Whether calculating how many moles of ammonia (NH₃) are in a solution or assessing nitrogen fixation rates in agroecosystems, nitrogen’s mass per mole anchors every calculation. In industrial chemistry, nitrogen’s mass determines flow rates in gas storage and distribution systems, critical for fertilizer plants and semiconductor manufacturing where even trace impurities affect performance.
A key application lies in environmental science.
Nitrogen compounds influence air quality, soil fertility, and climate change. Monitoring nitrogen oxides (NOₓ) in emissions relies on knowing their molecular weight. Atmospheric models tracking greenhouse gases integrate precise nitrogen molar mass to isolate nitrogen’s impact from other gases.
As lead researcher Dr. Marcus Lin explains, “Our ability to quantify nitrogen cycling depends on exact molar data—without it, predictions of ecosystem responses to climate shifts remain uncertain.”
Why Use 14.01 Instead of 14.007? The Pragmatics of Precision
Though the atomic mass of nitrogen is calculated at approximately 14.007 g/mol using modern mass spectrometry, the value 14.01 offers practical advantages.In teaching labs and routine analysis, rounding simplifies calculations without sacrificing acceptable accuracy. This standardized figure ensures consistency across textbooks, research papers, and industrial protocols. For instance, when calculating the nitrogen content in soil or sludge, a deviation of 0.001 g/mol may be negligible, but across thousands of data points, precision averts systematic errors.
The International Union of Pure and Applied Chemistry (IUPAC) reinforces this, advocating standardized values for reproducibility. As analytical chemist Dr. Elena Torres states, “Consistency in molar mass values preserves data integrity—a foundation without which science cannot scale.”
This pragmatic rounding does not diminish nitrogen’s scientific importance.
In pharmaceutical development, accurate molar mass determines dosage formulations. For air processing in medical SCBA (Self-Contained Breathing Apparatus), nitrogen’s weight per mole ensures safe oxygen delivery ratios. Every gram per mole counted—and every exact decimal applied—reflects a commitment to precision that safeguards human health and industrial safety.
Applications Across Key Domains
- Analytical Chemistry: Nitrogen’s molar mass underpins gravimetric and volumetric analysis, enabling precise quantification of organic and inorganic nitrogen compounds. - Agricultural Science: Measuring nitrogen availability in fertilizers and soil relies on molar mass-based moles to optimize crop nutrition and minimize environmental runoff. - Environmental Science: Tracking atmospheric nitrous oxide (N₂O)—a potent greenhouse gas—depends on nitrogen’s precise mass to model climate impacts.- Biotechnology: Nutrient metabolism modeling, enzyme kinetics, and metabolic pathway simulations use nitrogen molar mass to decode biochemical networks with fidelity.
From classroom labs to global climate assessment, the molar mass of nitrogen stands as a silent sentinel, ensuring data reliability and enabling cross-disciplinary breakthroughs. In an era where scientific accuracy drives innovation, nitrogen’s defining

Related Post

What Are Sinews? A Simple Definition You Need to Know
The Enigmatic Power of Amggvalues: Decoding Trend Analysis and Performance Metrics

From 39°C to F: The Science Behind the Thermal Threshold Shifting

Mycah Hatfield: The Athletes’ Wife in the Spotlight – Age, Height, and Life Behind the Headlines

