Which Statements About Protein Digestion Are Actually True? The Science Backed by Biology
Which Statements About Protein Digestion Are Actually True? The Science Backed by Biology
Protein digestion is a complex, highly orchestrated physiological process essential for harnessing the building blocks of life from dietary sources. From the moment food enters the mouth to the final absorption of amino acids in the small intestine, each phase of protein breakdown is precisely regulated by enzymes, pH environments, and specialized cellular machinery. Understanding which statements about this process stand true is critical not only for medical, nutritional, and research fields but also for everyday insights into optimal health and dietary choices.
This article dissects the factual core of protein digestion, evaluating common assertions with biological precision to distinguish truth from misconception.
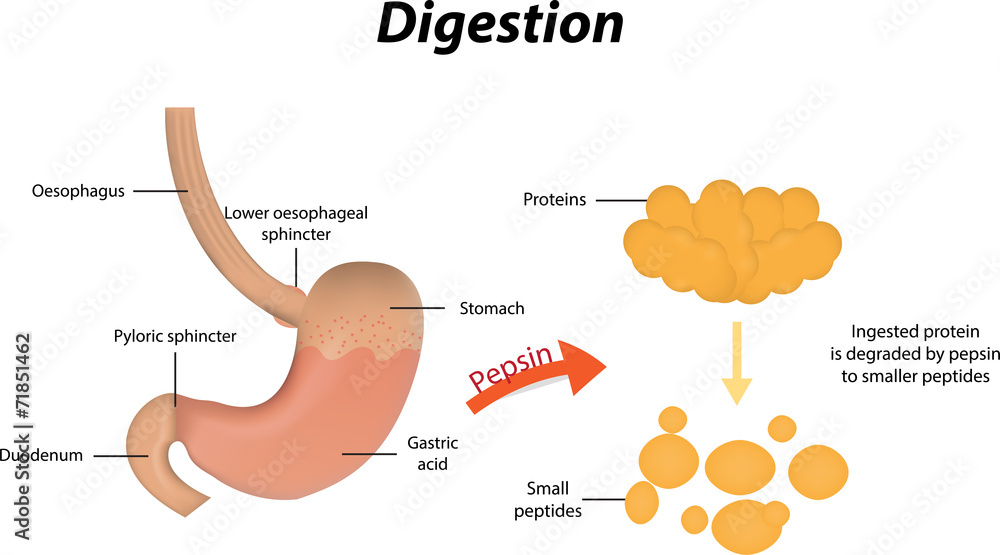
Protein digestion begins in the stomach, where the acidic environment unfolds the complex tertiary structures of dietary proteins, enabling the enzyme pepsin to cleave peptide bonds. This transformation is indispensable: native protein configurations are too compact for efficient breakdown.
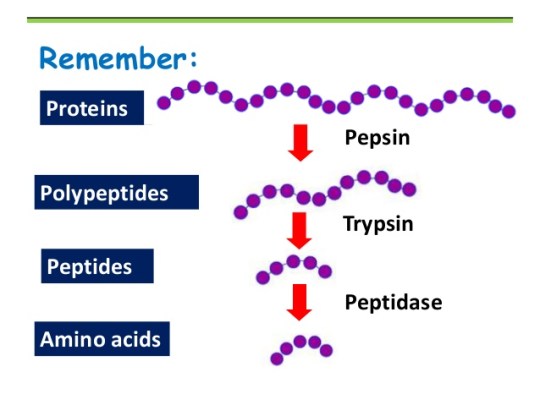
As digested fragments move into the duodenum—the primary site of digestion—pancreatic enzymes such as trypsin, chymotrypsin, and carboxypeptidase take over, systematically dissecting proteins into smaller peptides and individual amino acids. Unlike the static view of digestion once held by early science, modern research reveals this as a dynamic cascade where each enzyme acts in concert, guided by regulatory signals and substrate specificity. “The gastrointestinal tract functions not as a simple pipe, but as a precision bioreactor,” explains Dr.
Elena Marquez, a gastroenterologist at the University of Bologna. “Each stage is calibrated to maximize nutrient extraction while minimizing waste.” Here are the key truths underpinning the science:
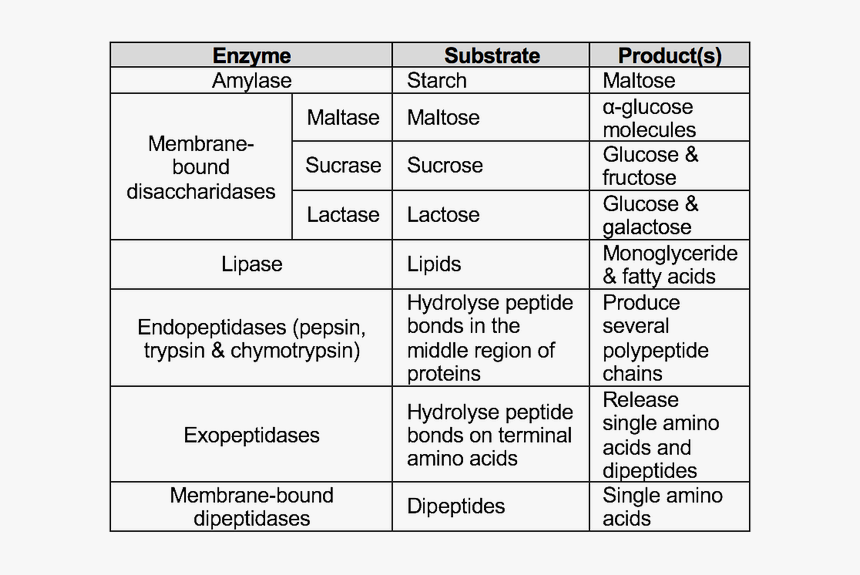
Protein Digestion Relies on a Sequential Enzymatic Cascade
Far from a single-step transformation, protein digestion unfolds across multiple stages, each governed by specific enzymes and optimal pH conditions. - **Stomach:** Pepsin, activated from pepsinogen by hydrochloric acid, initiates cleavage, fragmenting proteins into smaller polypeptides.- **Small Intestine:** The pancreas releases proteolytic enzymes—trypsin, chymotrypsin, and elastase—whose specificities determine how peptide chains are cleaved. - **Brush Border Peptidases:** Once remaining peptides reach the intestinal lining, brush border enzymes like aminopeptidases and dipeptidases finalize digestion, converting peptides into free amino acids ready for absorption. “This sequential breakdown ensures near-complete liberation of amino acids,” notes Dr.
Rajiv Patel, a biochemist specializing in digestive physiology. “Without this precision, one could lose up to 30% of dietary protein’s usable building blocks—a gap with measurable impacts on tissue repair and metabolic function.”
Enzymes Require Specific pH Environments to Function Optimally
The digestive system’s pH gradient is not random but a finely tuned environment critical for enzyme activation and stability. - **Stomach:** With a pH between 1.5 and 3.5, optimal for pepsin activity, acidic conditions unfold proteins and protect ingested microbes.- **Duodenum and Jejunum:** Enzymatic digestion flourishes in a near-neutral pH (6–7.4), where pancreatic enzymes operate at peak efficiency. - **Mismatched pH Disrupts Function:** If stomach acid is overly suppressed—particularly by proton pump inhibitors—trypsin and other enzymes fail to activate properly, resulting in incomplete protein breakdown and reduced amino acid availability. Conversely, alkaline shifts in the stomach (e.g., from vomiting or特定medications) inhibit pepsin, slowing digestion from the start.
The reliance on precise pH underscores why digestive disorders—such as Gastric Atrophy or chronic heartburn—pose dual risks: compromised digestion and diminished nutrient access. Maintaining digestive homeostasis remains a cornerstone of metabolic health.
Protein Source Influences Digestive Efficiency and Amino Acid Bioavailability
Dietary protein types—animal, plant, or purified supplements—differ structurally and chemically, affecting how thoroughly they are digested and absorbed. - **Animal Proteins (e.g., meat, eggs, dairy):** Rich in complete amino acid profiles and relatively resistant to denaturation, they generally yield higher digestibility—often exceeding 90%—due to favorable peptide structures that resist degradation until later stages.- **Plant Proteins (e.g., legumes, grains):** Frequently limited in essential amino acids like lysine or methionine; additionally, plant cell walls contain anti-nutrients (e.g., phytates, tannins) that inhibit protease activity, lowering effective digestibility to 70–85%. - **Hydrolyzed and Processed Proteins:** Supplements like whey isolate undergo pre-digestion (hydrolysis), allowing faster absorption—beneficial in clinical recovery or rapid muscle protein synthesis but potentially less satiating. Understanding these distinctions empowers informed nutritional choices, particularly for athletes, elderly individuals, or those managing GI conditions where digestibility is paramount.
The Final Stage: Absorption of Amino Acids in the Small Intestine
Once proteins are reduced to free amino acids and small dipeptides, absorption occurs almost exclusively in the示范小肠(小肠)长段, particularly the jejunum. - **Sodium-Dependent Transporters:** Most amino acids enter enterocytes via sodium-coupled symporters or antiporters, requiring energy and regulation. - **Amino Acid Variability:** Neutral and acidic amino acids use distinct transporters; small dipeptides (e.g., alanine-glycine) may use peptide transporters (Pept1), bypassing full hydrolysis but still demanding precise binding.- **Transport to Systemic Circulation:** After uptake, amino acids fuel protein synthesis, neurotransmitter production, and metabolic pathways or enter the liver for further processing before reaching peripheral tissues. “You eat to rebuild, not just to satisfy hunger,” reminds Dr. Marquez.
“The efficiency of this final step determines whether nutrients fuel energy, repair, or immune defense—or are squandered.”
Dysfunction in dendritic amino acid transport, whether due to genetic mutations, inflammation, or nutritional deficits, can impair recovery and compromise muscle and organ integrity over time. This highlights why balanced, high-quality protein intake paired with digestive health is nonnegotiable for physiological resilience.
Common Myths Debunked
Several widespread beliefs misrepresent protein digestion and defy scientific evidence: - **Myth:** Raw protein is better absorbed than cooked. Fact: Cooking denatures proteins, making peptide bonds more accessible to digestive enzymes—generally enhancing, not reducing, digestibility.- **Myth:** High-fiber diets block protein digestion. Fact: Fiber potently affects transit time but does not block enzymatic action; instead, fiber supports gut health, indirectly enabling consistent digestive function. - **M

Related Post

Jennifer Garner and Ben Affleck’s Wedding: When Hollywood Elite Wove Love and Legacy Into a Media Event

Anime Card Clash Codes 2025: June Active Code Shatters Expectations and Rewrites Roblox Rewards Gameplay

Former NFL Michael Lavaughn Robinson Football: Unraveling Legacy & Lasting Impact

Melanie Lynn Clapp: Architect of Ethical Innovation in Technology and Media

