What You Never Knew About the Gift of Boron: The Secret Identity of Group 17 Elements
What You Never Knew About the Gift of Boron: The Secret Identity of Group 17 Elements
Among the periodic table’s most intriguing groups lies Group 17 — the halogens — whose elements possess distinct chemical behaviors shaped by their singular electron configurations. Known formally as *halogens*, derived from the Greek word meaning “salt-bearing,” this group includes fluorine, chlorine, bromine, iodine, astatine, and the yet unobserved tennessine. Together, these elements share a defining trait: they each possess seven valence electrons, making them one electron short of a stable octet.
This structural detail underpins their explosive reactivity, metallic character in heavier members, and critical biological roles — collectively earning them the moniker “Group 17 Elements.” Their collective nomenclature reveals far more than a label — it uncovers a unified chemical philosophy rooted in electron dynamics.
Core Traits Uniting the Halogens: The Electron Configuration Blueprint
At the heart of Group 17’s chemical identity lies a consistent electron configuration: ns² np⁵ — meaning a full outer energy level with seven electrons. This configuration creates a profound electron-deficiency, driving each halogen to gain one electron and achieve the stability of a noble gas.As the Nobel laureate Linus Pauling noted, “The halogens are among the most electronegative and reactive elements — their hunger for an additional electron defines their chemistry.” This insatiable drive for electron balance fuels key reactions, including eager ionic transfers and fierce oxidative processes. The electron-poor octet explains why fluorine, the most electronegative element, leads the group. Its ability to attract electrons with unmatched fervor underpins its role in water purification, pharmaceuticals, and advanced materials.
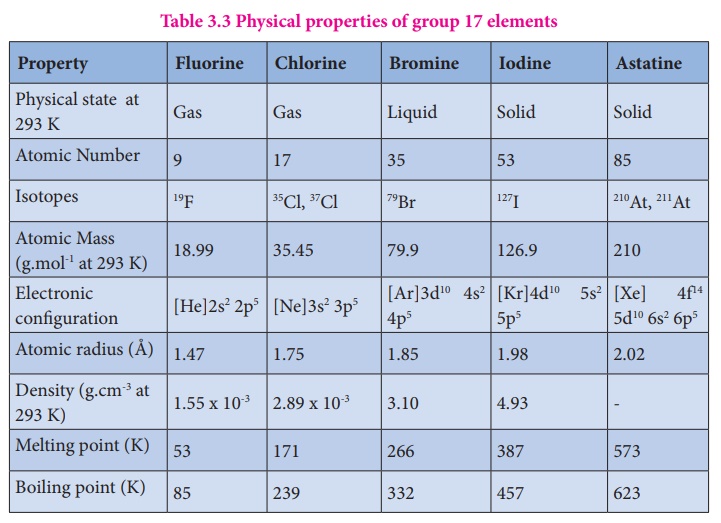
Lighter halogens like chlorine and bromine, though far less electronegative, still display strong oxidizing behavior, forming salts and disinfectants vital to public health. Even iodine, often overlooked, plays an essential biochemical role in thyroid hormone synthesis. Group 17 elements display a clear trend in physical state and reactivity from fluorine to astatine — the rarest and most exotic, with fewer known isotopes.
Fluorine and chlorine exist as diatomic gases at room temperature, while bromine is a red-brown liquid, and iodine a violet solid. Astatine, theoretically predicted but scarcely isolated, remains one of the rarest naturally occurring halogens, prized for rare nuclear applications.
Harnessing the Power: Industrial and Biomedical Applications of Group 17 Elements
The practical significance of Group 17 elements spans everyday life and cutting-edge science.Fluorine, often celebrated for unlocking blockbuster pharmaceuticals and water fluoridation, underpins glass, Teflon, and high-performance batteries. Chlorine dominates disinfection in drinking water and swimming pools, eliminating pathogens with unparalleled efficiency. Bromine compounds serve as flame retardants in furniture and electronics, while iodine remains indispensable in medical diagnostics and salon treatments.
Emerging technologies continue to unlock new roles: radioactive iodine isotopes target thyroid cancers, aluminum bromide functions as a catalyst in refining, and ongoing research explores astatine’s potential in targeted radiotherapy. “The halogens offer one of chemistry’s most versatile toolkits,” observes synthetic chemist Dr. Elena Marquez.
“Each element, with its nuanced reactivity, expands what’s possible in medicine, materials, and environmental protection.” Weight and radioactivity define the lighter members: fluorine and chlorine are stable, abundant in Earth’s crust; bromine remains moderately reactive; iodine, though essential, poses toxicity challenges at high doses. Astatine’s extreme scarcity and radioactivity make it more a subject of study than industry use — yet even its potential highlights the group’s untapped promise.
Synthesis and Challenges: The Future of Group 17 Elements
Despite decades of study, Group 17 elements continue to surprise.Tennessine, the most recently synthesized and least understood, emerged from decades of nuclear research — synthesized in particle accelerators through fleeting nuclear fusion. Its fleeting presence underscores both the havoc these elements can unleash and the precision required to study them. Environmental and health concerns further complicate their use.
Chlorinated solvents contribute to ozone depletion; brominated flame retardants accumulate in ecosystems. Yet, innovation persists — green chemistry initiatives seek safer alternatives, while radioisotopes render diagnostically powerful yet minimally invasive tools. Looking ahead, the halogens embody chemistry’s dual nature: volatile yet indispensable, reactive yet remarkably predictable when understood.
Their electron-configured blueprint guides discovery, from pharmaceuticals to nuclear medicine. Group 17 elements — the halogens — are not merely a periodic family; they are a dynamic force shaping science, health, and technology.
Through their seven electrons and unrelenting pursuit of stability, Group 17 elements reveal a universal principle: chemical identity emerges from electron dynamics.
As research evolves, so too does our understanding — illuminating the quiet but profound influence of boron’s “cousins” in the tapestry of matter. In every reaction, every innovation, and every safeguard, the halogens prove that elemental destiny is written not just in symbols, but in the rhythm of electrons seeking balance.



Related Post

Is Las Vegas in Nevada Exploring the Metro Area?

Bears vs Lions: A Contrast in Power, Precision, and Playmaking — Bears Key Players vs. Lions' Atomic Attackers

Q3 2025 marks a pivotal quarter for global markets, economic resilience, and transformative industry shifts—analysis reveals a story of cautious optimism amid evolving dynamics.

Angie Hendershot’s Life Rose in Bright Tidbits: Age, Height, and the Steady Presence of Her Husband

