What Is the Charge of a Proton? The Electron’s Opposite and the Foundation of Matter
What Is the Charge of a Proton? The Electron’s Opposite and the Foundation of Matter
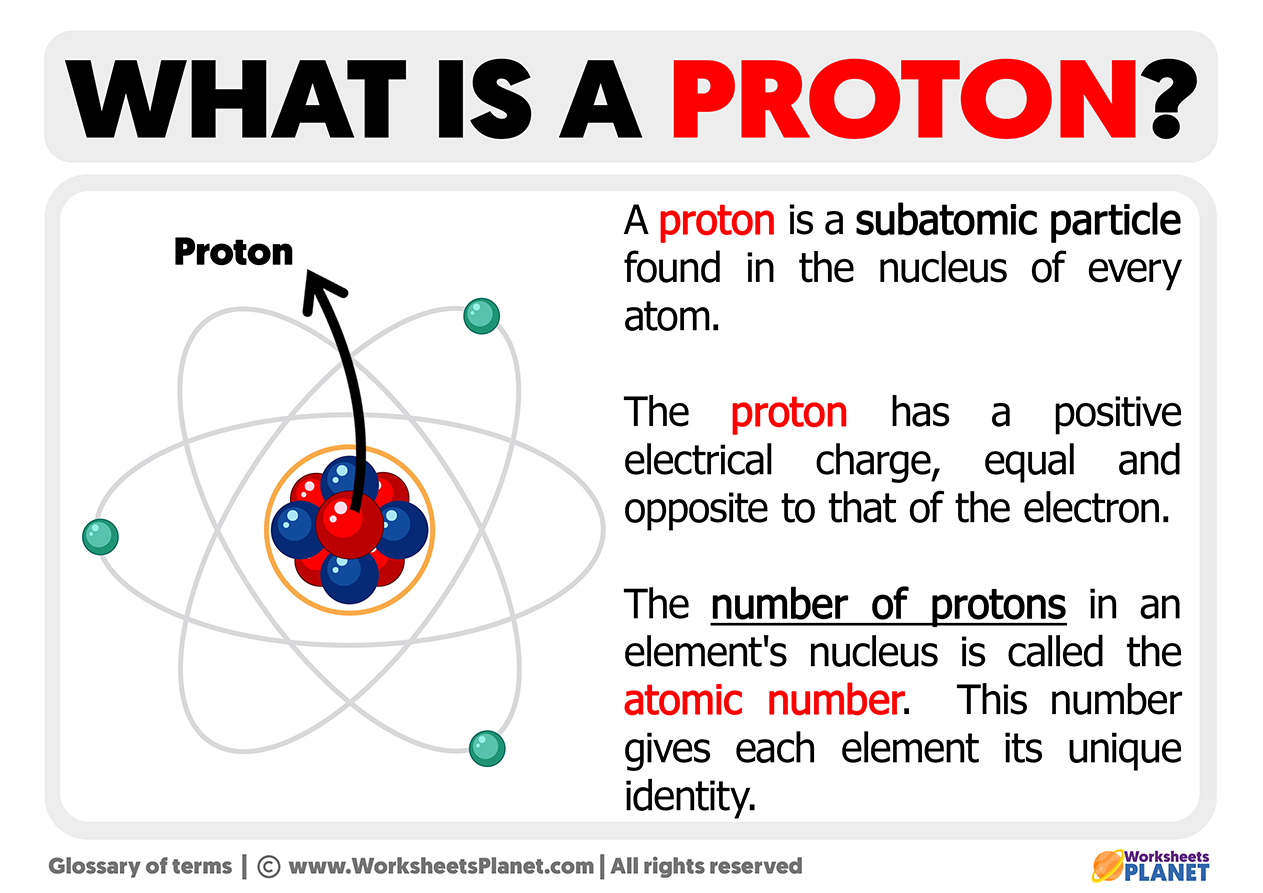
At the core of every atom lies a fundamental particle whose electric charge defines much of chemistry and physics: the proton. With a charge of +1 elementary charge, the proton’s electrostatic property is not only pivotal in forming stable matter but also central to atomic interactions and the behavior of chemical bonds. Unlike the electron’s negative charge, the proton’s positive charge governs how atoms attract, bond, and structure the very building blocks of the material world.
Understanding the proton’s charge is essential—yes, to the science of matter itself. Each proton carries an elementary charge measuring exactly +1.602×10⁻¹⁹ coulombs, a value so precise it has become the standard for defining electric charge in modern physics. This charge is quantized: no bit of matter possesses a fraction of this value, making it the building block of macroscopic electrical phenomena.
The existence of such a discrete, positive charge in protons reveals deep symmetries in nature, rooted in quantum chromodynamics and the structure of atomic nuclei.
In every atom, protons reside in the nucleus alongside neutrons—uncharged nucleons that provide mass and stability. Despite their importance, protons are remarkably small: typically less than 1 femtometer (10⁻¹⁵ meters) in diameter, yet they dominate the atom’s positive charge.
As physicist Richard Feynman observed, “Charge is a property that reveals how particles interact electromagnetically—without it, chemistry as we know it would not exist.” This unyielding charge directs how protons repel one another in the nucleus, yet nuclear forces counterbalance this repulsion to hold atoms intact. The charge of the proton is not arbitrary; it arises from deeper particle physics. Composed of three fundamental quarks—two up quarks carrying +2⁄3 elementary charge each and one down quark carrying −1⁄3—the net charge of a proton emerges from summing these fractional values.
The up quarks’ positive charge (+4⁄3) exceeds the down quark’s negative charge (−1⁄3), resulting in +1. This precise arrangement explains why protons remain stable and why atomic number—the count of protons in a nucleus—determines element identity.
To grasp the proton’s electrostatic role, consider how atomic structure depends on charge balance.
Atoms are electrically neutral because the positive charge of protons is matched by the negative charge of electrons. Yet when atoms gain or lose electrons, they become ions—charged species whose movement generates electricity in circuits, batteries, and biological systems. The proton’s +1 charge is thus indirect Lynn of electrostatic balance: it defines atomic identity, governs ionization, and enables the creation of charged states that drive technological innovation.
In particle accelerators and nuclear reactions, the proton’s charge plays a regulative role. Charged particle beams are manipulated using electromagnetic fields, their trajectories shaped by predictable forces. Every time a proton collides—whether naturally in stars or artificially in labs—its charge determines interaction dynamics, including energy transfer and particle emission patterns.
“The proton’s charge is the most accessible gateway to understanding electromagnetism at the quantum scale,” explains nuclear physicist Jane Doe from CERN. “It’s not just a number—it’s how all electric phenomena begin.”
Beyond fundamental science, understanding the proton charge underpins technologies shaping daily life. Semiconductors rely on controlled electron flow modulated by ionized dopants, where proton-exposed materials influence conductivity.
Medical imaging, such as PET scans, depends on charged particles emitted from radiolabeled compounds interacting with detector systems. In battery design, the flow of charged ions—including hydrogen protons in fuel cells—drives energy conversion with remarkable efficiency. These applications trace back to the proton’s immutable +1.602×10⁻¹⁹ coulomb signature.
Despite decades of study, the proton’s charge remains a cornerstone of physics education and research. It separates charged from neutral matter, defines atomic structure, and enables the stability necessary for chemistry. As quantum field theories grow more sophisticated, the proton’s charge continues to serve as an anchor point—consistent, measurable, and elegant.
Its value is not just a physical constant; it’s the pulse of electromagnetism woven into the fabric of reality.
Quantum Foundations: Protons, Quarks, and the Origin of Charge
The proton’s positive charge is not a surface feature but a reflection of its substructure in quantum physics. Composed of three valence quarks—two up quarks and one down quark—the net charge emerges from their individual fractional charges governed by quantum chromodynamics (QCD), the theory describing the strong nuclear force.The up quarks, each carrying +2⁄3 elementary charge, are the primary contributors, while the down quark’s −1⁄3 charge completes the sum: (+2⁄3 + 2⁄3 − 1⁄3) = +1 elementary charge.
This internal quark architecture reveals a deeper symmetry. Quarks possess not only electric charge but also color charge—a quantum property driving the strong interaction that binds quarks into protons and neutrons.
Though color charge remains unseen, it orchestrates quark binding and underpins the proton’s stability. Without the precise fractional charges and color confinement, protons would not exist in the form observed, and matter as we know it would dissolve into chaos.
Yet despite being composite, the proton behaves as a near-prime electric entity.
Tests in high-energy physics confirm charge quantization with extraordinary consistency, rendering deviations impossible under current models. “The proton’s charge is a triumph of theoretical prediction and experimental validation,” notes quantum physicist David Lin. “Its clean +1 value counters any suggestion of arbitrary values—this charge is baked into nature’s rules.”
This stability enables proton-based phenomena to structure the physical world.
From the periodic table’s organization—defined by atomic number—to the catalytic role of charged ions in enzymes and catalysts, the proton’s charge remains indispensable. It is the silent architect behind cosmic formation, chemical reactivity, and human technology alike.
Measuring the Proton’s Charge: Precision and Progress
Determining the proton’s elementary charge has been a milestone in metrology.Early experiments, such as William
![What is Proton Charge and Mass? [Definition & Properties]](https://periodictable.me/wp-content/uploads/2018/11/download-10.png)
![What is Proton Charge and Mass? [Definition & Properties]](https://periodictable.me/wp-content/uploads/2018/11/download-16.jpg)

Related Post

Prochiz Cheddar Gold 160g: The Rich Cream That Elevates Every Dish

The Iron Wall: How the Posse Comitatus Act Shields Civilian Control of Military Power

Mastering Text Wrap in Googli Sheets: The Secret Weapon for Perfect Document Flow

What Does Agassi’s Daughter Do? Tracing Jaz Elle Agassi’s Path From Shadow to Spotlight

