What Is a Main Group Element? The Core of Chemical Organization
What Is a Main Group Element? The Core of Chemical Organization
At the heart of every chemical compound’s structure lies a fundamental concept: the main group element, the foundational building block that defines classification, bonding behavior, and reactivity. These elements, occupying key positions in the periodic table, serve as the backbone for understanding chemical relationships across science and industry. From sodium’s electrifying role in salts to silicon’s pivotal part in technology, main group elements organize matter in predictable, predictable, and profoundly useful ways.
But what exactly defines these essential players, and why do they hold such authority in chemistry? This article unpacks the essence of main group elements—where they are found, how they function, and why their placement on the periodic table matters.
The Definition and Positioning of Main Group Elements
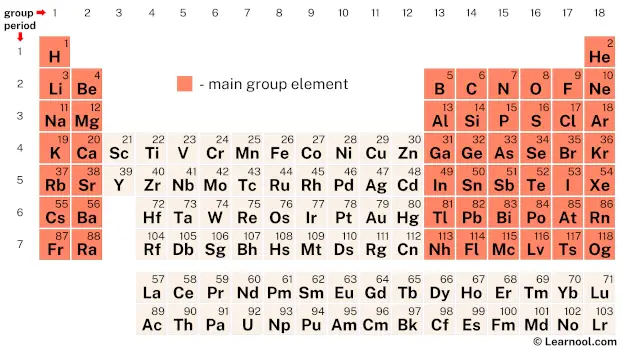
Main group elements are defined as those occupying Groups 1, 2, and 17–18 of the periodic table—elements whose valence electron configuration reliably determines their chemical behavior. These elements include alkali metals, alkaline earth metals, halogens, and the noble gases, with lanthanum and actinium occasionally included in expanded definitions.
Consistent across the board, main group elements exhibit predictable ionic or covalent bonding patterns shaped by eight electrons in their outermost shell, a capacity formalized by the octet rule. This structural regularity enables scientists to anticipate how these elements interact, replace one another in compounds, and contribute to both natural and synthetic materials. “The main group elements represent a bridge between atomic simplicity and chemical complexity,” explains Dr.
Lena Marquez, a periodic system expert at the International Union of Pure and Applied Chemistry (IUPAC). “Their well-defined electron behavior makes them invaluable in predicting reactivity and forming stable compounds.”
Structural Characteristics and Universal Trends
Each main group element displays a distinct set of physical and chemical traits tied to its position in the periodic table. Elements in Group 1—such as lithium, sodium, and potassium—are soft, highly reactive metals with low ionization energies, easily losing one electron to achieve noble gas configurations.
In contrast, Group 17 halogens—including fluorine, chlorine, and iodine—are potent oxidizing agents, aggressively gaining one electron to complete their octet. Meanwhile, Group 18 noble gases like helium, neon, and argon remain chemically inert due to their full valence shells, making them stable yet carefully contained in nature. With every ascending group, atomic radius increases while ionization energy decreases—factors that directly influence reactivity.
These predictable trends allow chemists to map out reaction pathways, safeguard handling protocols, and engineer novel materials based on elemental behavior.
Core Roles in Chemistry and Technology
Main group elements are not merely academic constructs; they drive countless applications across industries. Classified as Group 1 and Group 2 metals, alkali and alkaline earth elements serve critical roles: sodium and chlorine form sodium chloride, a compound essential not just in nutrition but in industrial plastic production; calcium underpins bone health and acts as a key ingredient in cement, reinforcing concrete with silica. Transitioning to nonmetals, Group 14’s carbon forms the backbone of organic chemistry and nanotechnology; silicon, from Group 14, powers modern electronics through semiconductors.
Even the halogens transform medicine and industry—iodine in antiseptics, fluorine in durable polymers, chlorine in water purification. Beyond utility, main group elements illuminate fundamental scientific principles, from electron transfer in batteries to catalytic activity in green chemistry. “Understanding these elements is like holding a Rosetta Stone for chemical bonding,” notes Dr.
Raj Patel, materials scientist at MIT. “Their predictable behavior enables innovation across engineering, medicine, and environmental science.”
Variability Within the Main Group: Exceptions and Nuances
While main group elements follow broad patterns, notable exceptions reveal the complexity beneath their simplicity. Lithium, though Group 1, resists extreme reactivity more than sodium or potassium, a quirk linked to its small atomic size.
Similarly, boron in Group 13 defies strong covalent bonding expectations, exhibiting semi-metallic traits due to its electron-deficient structure. Even noble gases, traditionally inert, participate in rare compounds—xenon forms cryogenic liquids used in anesthesia, while krypton contributes to lighting technology. “These nuances challenge the neat boxes we draw around periodic categories, but they enrich our understanding,” highlights Dr.
Elena Rossi, organometallic chemist at Stanford University. The existence of such variants underscores that while main group elements form a structured framework, nature occasionally introduces strategic departures that expand their functional range.
Periodic Table Positioning and Elemental Identity
The main group elements are precisely located on the periodic table’s vertically aligned columns—Groups 1, 2, and 17–18—where their grouping reflects shared valence electron behavior. This placement is not arbitrary: elements in the same group share analogous chemical properties, as seen in chlorine’s high electronegativity or magnesium’s tendency to form +2 ions.
The periodic table’s design thus crystallizes the connection between atomic structure and function. “Position matters because it reveals identity,” says Dr. Ian Thompson, period theory analyst at the Royal Society of Chemistry.
“When elements align by valence, chemistry becomes predictable—enabling synthesis, innovation, and discovery in fields from pharmaceuticals to renewable energy.” This spatial organization transforms the table from a mere chart into a functional roadmap of the material world.



Related Post

Direct2Hr Login: Revolutionizing HR Access with Secure, Seamless Employee Self-Service

The Age Of Adaline Cast

Corey Jackson Football: The Rise of a Defensive Powerhouse Redefining Modern Lines

Was 50 Cent Ever Married? Uncovering the Truth Behind the Rap Reputation

