What Is a Conjugate Acid? The Hidden Partner in Acid-Base Chemistry
What Is a Conjugate Acid? The Hidden Partner in Acid-Base Chemistry
At the heart of acid-base chemistry lies a foundational concept that shapes how scientists understand proton transfer: the conjugate acid. Often overlooked but essential, a conjugate acid is not merely a relic of chemical reactions but an active participant in the dance of H⁺ ions. Defined as the species formed when an acid donates a proton, the conjugate acid completes a dynamic equilibrium central to pH, buffering, and biological function.
As chemist Peter Atkins observes, “Acids and their conjugate bases are two sides of the same proton-sharing coin.” Understanding this relationship reveals deeper insight into chemical stability, industrial processes, and even life’s molecular machinery.
The Proton Exchange: Defining the Conjugate Acid
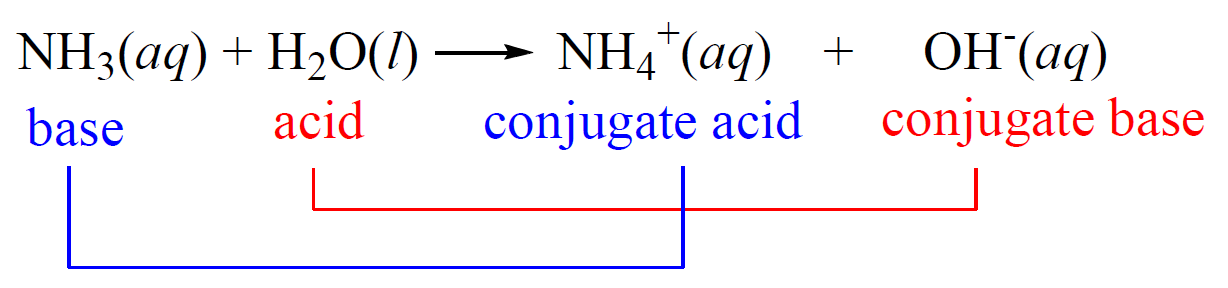
A conjugate acid originates when a classical acid releases a proton (H⁺), transforming into a base by accepting that hydrogen ion. This transformation is governed by the Brønsted-Lowry theory, which defines acids as proton donors and bases as proton acceptors.When an acid donates H⁺, it becomes its corresponding conjugate base, while the newly formed H⁺ accepts electrons—forming a conjugate base. The reversibility of this exchange underscores the acid-base equilibrium, encapsulated in equations like: Acid H₃O⁺ ⇌ H⁺ + Conjugate base HA⁻ This relationship means every acid has a unique conjugate base, and vice versa, forming complementing pairs that stabilize chemical reactions. For instance, when hydrochloric acid (HCl) donates a proton, it becomes chloride ion (Cl⁻), the conjugate base.
“The conjugate acid-base pair exists in constant flux, with strengths tied by equilibrium constants,” explains academic resource Chemistry LibreTexts. The relative strengths of an acid and its conjugate base determine whether a solution favors proton donation or acceptance—critical in predicting reaction direction.
Key Properties and Nomenclature of Conjugate Acids
Conjugate acids exhibit predictable chemical behavior shaped by their molecular structure.Unlike regular acids, they typically increase in stability after accepting H⁺—a reflection of increased positive charge that nucleophiles readily stabilize. Many conjugate acids appear as common ions: ammonium (NH₄⁺), hydronium (H₃O⁺), and hydrogen sulfate (H₂SO₄⁻) are ubiquitous in aqueous systems. These ions influence pH directly; hydronium (H₃O⁺) defines acidity in water, where pH = –log[H₃O⁺].
Naming conjugate acids follows systematic rules: begin with the uncharged parent acid, then drop the “-ic” ending and replace it with “-ate” to indicate the deprotonated form. However, the conjugate base receives primary emphasis in naming due to its basic nature. For example, sulfuric acid (H₂SO₄) forms sulfuric acid itself as the acid, while its conjugate base, hydrogen sulfate (HSO₄⁻), is recognized in buffers and industrial processes.
“The conjugate acid often plays the more chemically active role, especially in catalysis and biological pathways,” notes the Handbook of Acid-Base Chemistry.
Real-World Impact: Where Conjugate Acids Shape Science and Industry
Conjugate acids are not confined to textbooks—they drive vital processes across chemistry, biology, and industry. In biological systems, proton equilibria mediated by conjugate acid-base pairs maintain pH homeostasis.The carbonic acid (H₂CO₃)/bicarbonate (HCO₃⁻) system, for example, regulates blood pH, shifting between H₂CO₃ and HCO₃⁻ to stabilize acidity—a balance so precise that disruptions can threaten health. In industrial applications, conjugate acids inform water treatment, pharmaceutical formulation, and battery chemistry. Hydronium ions generate acidity in contaminated water, prompting neutralization with conjugate base treatments like sodium bicarbonate (NaHCO₃).
In lithium-ion batteries, the oxide-derived conjugate acid influences ion transport and efficiency. “Understanding conjugate acid behavior allows precise control of reaction environments,” says Dr. Elena Martinez, a physical chemist.
“From enzyme function to material synthesis, this pairing is chemistry’s silent architect.”
The conjugate acid remains a cornerstone of understanding proton behavior—central to pH, reactivity, and stability. Recognizing its role transforms abstract theory into practical insight, empowering innovations from medical science to sustainable technology. In every reaction where H⁺ shifts, the conjugate acid stands ready, completing the proton exchange cycle that defines acid-base chemistry.
In Summary: The Conjugate Acid’s Defining Role in Chemical Equilibrium



Related Post

Gaby Gardez Unlocks the Science of Flavor: How One Palate Transformed Culinary Innovation

A Detailed Analysis: Bruno Genesio and Tanguy Coulibaly Illuminate the Interplay of Innovation and Strategy in Modern Business

What Is Signposting in Speech? Mastering the Art of Clear Communication

The Quiet Stability Behind Sandra Bullock’s Marriage: Insights into the Life of a Hollywood Icon with her Longtime Spouse

