Weight Molecular Weight: The Silent Determinant Shaping Chemistry, Pharmaceuticals, and Life Itself
Weight Molecular Weight: The Silent Determinant Shaping Chemistry, Pharmaceuticals, and Life Itself
Weight molecular weight—often overlooked in casual conversation—plays a foundational role in science and industry, governing everything from how molecules interact to the efficacy and dosage of life-saving drugs. It is far more than a numerical value on a periodic table; it is the quantitative bridge between atomic composition and macroscopic behavior, influencing solubility, density, phase transitions, and biological activity. With mass numerically equal to the sum of atomic weights (weighted by each element’s atomic mass), molecular weight dictates how matter behaves under different conditions, making it a critical parameter in chemistry, pharmacology, materials science, and beyond.
Molecular weight directly influences a molecule’s physical properties: larger, heavier molecules tend to be less volatile, have higher boiling points, and exhibit greater viscosity. For example, propylene (C₃H₆, molecular weight ~42 g/mol) evaporates more readily than butane (C₄H₁₀, MW ~58 g/mol), despite both being alkenes. This principle extends to polymers—polystyrene’s high molecular weight (~104,000 g/mol) produces rigid plastics, while lower molecular weight variants yield pliable foams.
Such distinctions are not theoretical; they underpin manufacturing processes and material design across global supply chains. In pharmaceutical development, molecular weight is a regulatory compass. Drugs must balance potency with bioavailability, a challenge deeply tied to molecular size.
The Lipinski Rule of Five—a cornerstone in drug design—states that for oral drugs, molecules under 500 daltons (a proxy derived from molecular weight) are more likely to be absorbed efficiently. A 2021 study in *Nature Reviews Drug Discovery* emphasized that higher-than-usual molecular weight often correlates with reduced cellular penetration, limiting effectiveness. Yet, innovation pushes boundaries: if carefully managed, larger molecules—such as monoclonal antibodies—leverage molecular weight to enhance target specificity, albeit requiring injection rather than oral delivery.
In manufacturing and materials engineering, precise control of molecular weight is non-negotiable. Polymers like polyethylene terephthalate (PET), crucial for beverage bottles and textiles, rely on regulating MW to tune clarity, strength, and heat resistance. A narrow molecular weight distribution ensures consistent product quality, while broader distributions may compromise mechanical performance. Similarly, in specialty chemicals—from agricultural formulations to coatings—adjusting MW alters viscosity, adhesion, and drying time, demonstrating how molecular weight acts as a master variable in formulation science. Even in energy systems, molecular weight shapes outcomes. High molecular weight hydrocarbons dominate fuels like μgasoline, prized for their energy density. Conversely, lighter molecules in hydrogen storage systems face challenges due to weak intermolecular forces, prompting research into novel compounds with optimized molecular profiles. Each application underscores molecular weight not as a static number, but as a dynamic determinant of function. Why Molecular Weight Matters in Pharmacokinetics
Molecular weight directly governs how drugs distribute and eliminate from the body.
Smaller molecules (e.g., penicillin, MW ~334 g/mol) diffuse readily into tissues and cross cell membranes, ensuring rapid onset. Larger molecules, however, face diffusion barriers—targeting the brain, for instance, often requires bypassing the blood-brain barrier or using delivery systems tailored to molecular size. The clinical impact is profound: does a drug’s molecular weight permit sublingual, injectable, or oral administration?
Does it accumulate in fat or remain in blood? These questions, rooted in MW, determine dosing regimens and therapeutic windows. Data from the FDA highlights this: small-molecule drugs (usually <500 Da) average oral bioavailability of 60–90%, while larger biologics often achieve lower, unpredictable uptake—justifying the development of antibody-drug conjugates designed to navigate size limitations.
The actual measured molecular weight may deviate from theoretical values due to loadings, isomers, and formation of aggregates. Analytical techniques like mass spectrometry and osmotic pressure measurements resolve this complexity, but understanding the contributing factors is essential for scientific accuracy. Weighted molecular weight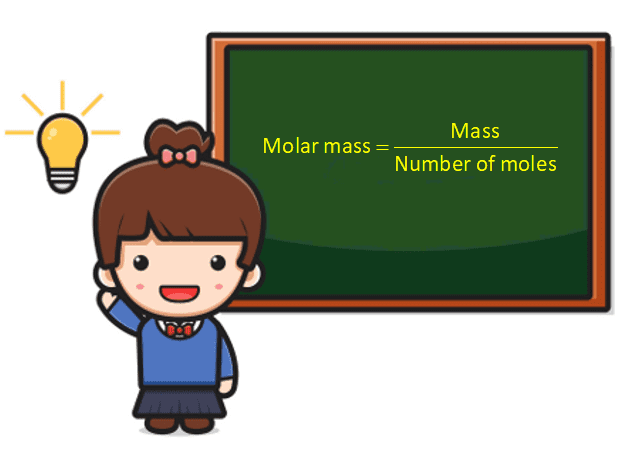


Related Post

Manatee County Jail Unleashes Transparency: Real-Time Inmate Mugshots Now Available via Public Search

Unlocking Ecological Insight: How Zuur et al.’s Framework Transforms Ecological Data Analysis
Bradenton Mugshots Reveal Charged Man After Dramatic Shooting During Domestic Conflict Involving Girlfriend, Ex-Boyfriend & Law Enforcement

Find Your Nearest McDonald’s in Seconds: Use McDonald’s Near Me to Locate the Closest Location Today

