Unraveling the Molecular Formula of Carbonate: Chemistry, Compounds, and Critical Applications
Unraveling the Molecular Formula of Carbonate: Chemistry, Compounds, and Critical Applications
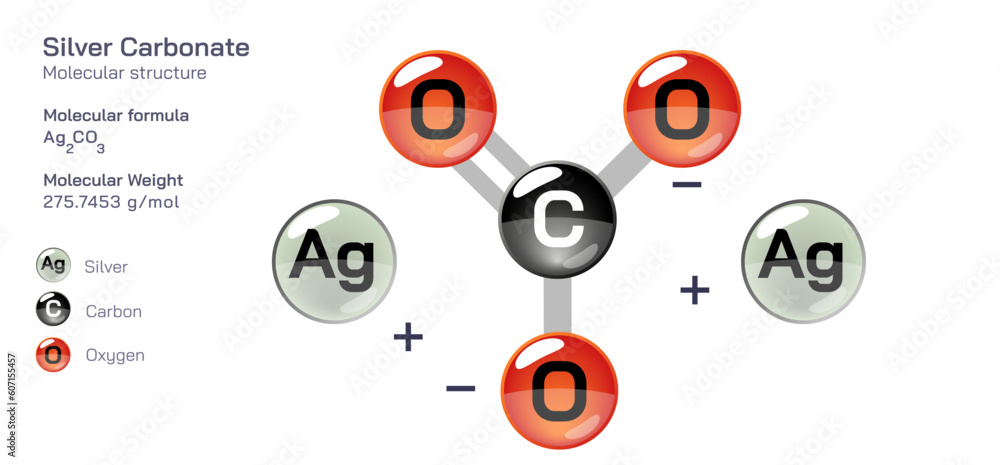
Each week, countless chemical compounds shape life, industry, and technology—yet few engage as dynamically in industrial processes and environmental systems as carbonate species. At the heart of this chemistry lies the molecular formula of carbonate, a neighborly ion defined by its unique combining of carbon, oxygen, and charge, with fundamental implications across science and engineering. Carbonate, formally represented as \( \text{CO}_3^{2-} \), emerges from a central carbon atom bonded to three oxygen atoms in a trigonal planar arrangement.
This structure confers both stability and reactivity, making it a cornerstone in fields ranging from geochemistry to pharmaceuticals.
The molecular formula of carbonate—\( \text{CO}_3^{2-} \)—is more than a symbolic shorthand; it encapsulates a powerful ion with distinctive physical and chemical properties. Composed of one carbon atom covalently bonded to three oxygen atoms, the molecule reflects resonance stabilization, where electron density spreads across the three C–O bonds, reducing charge concentration and enhancing stability.
This delocalization contributes to carbonate’s ability to dissolve readily in water—a behavior critical to marine biology and industrial processes alike. “The carbonate ion’s resilience stems from its resonance hybrid,” explains Dr. Elena Markova, a geochemist at Stanford University.
“This balance between covalent bonding and distributed charge enables carbonate to participate actively in carbon cycling across oceans, soils, and engineered systems.”
The formation of carbonate ion occurs through the deprotonation of carbonic acid (\( \text{H}_2\text{CO}_3 \)), a weak acid formed when carbon dioxide (\( \text{CO}_2 \)) dissolves in water. In equilibrium, \( \text{H}_2\text{CO}_3 \) partially dissociates: \[ \text{H}_2\text{CO}_3 \rightleftharpoons \text{H}^+ + \text{HCO}_3^- \quad \text{(bicarbonate)} \] \[ \text{HCO}_3^- \rightleftharpoons \text{H}^+ + \text{CO}_3^{2-} \quad \text{(carbonate)} \] This sequence, governed by pH and temperature, illustrates how carbonate emerges naturally in natural waters and man-made systems. Each dissociation step releases a proton, lowering acidity and shifting equilibrium—critical for buffering pH in ecosystems.
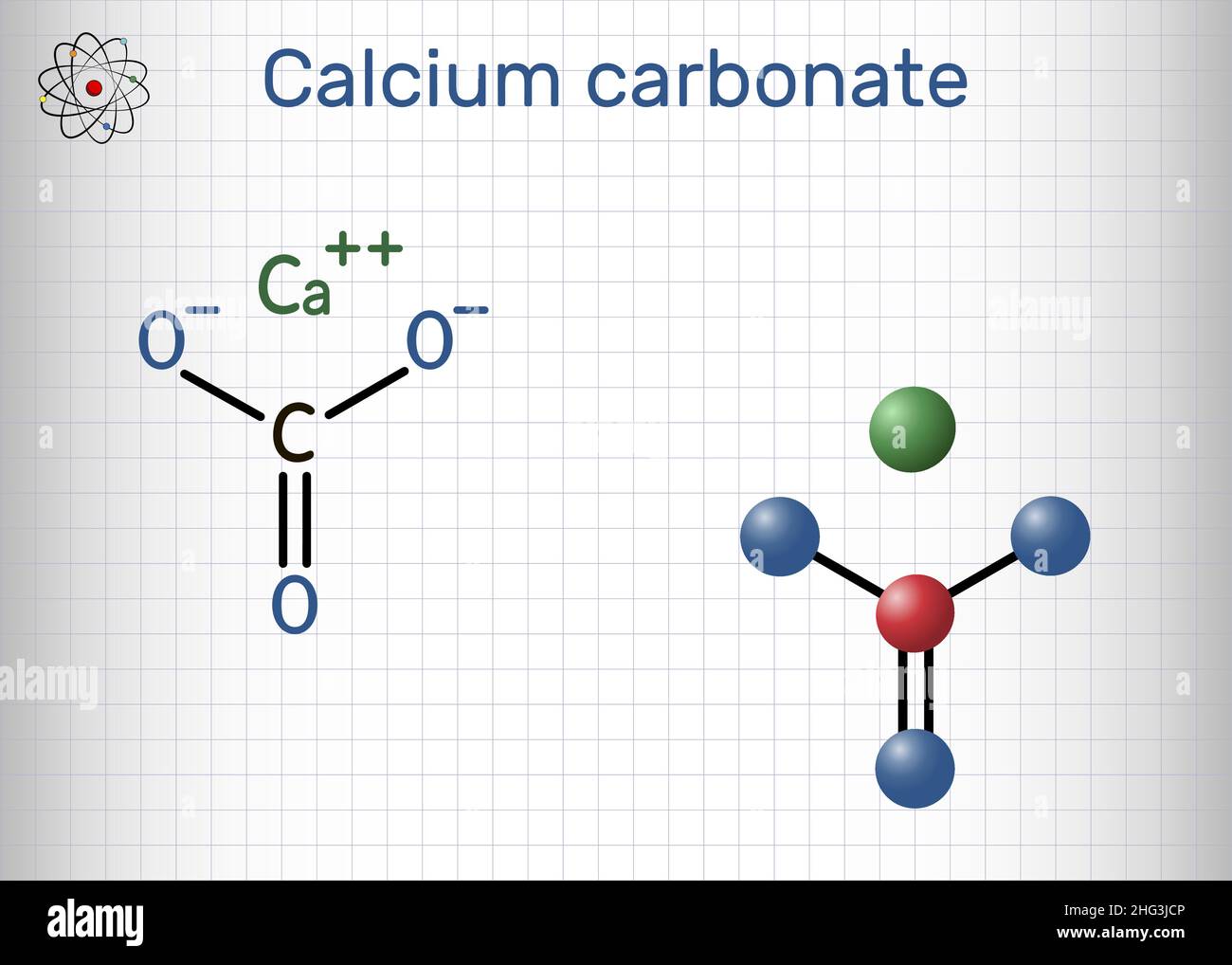
Carbonate’s utility extends far beyond aqueous solutions. Its molecular structure makes it an indispensable building block in material science. Calcium carbonate (\( \text{CaCO}_3 \)), for example, forms the primary component of limestone, seashells, and coral reefs, serving as nature’s long-term carbon reservoir.
Industrially, calcium carbonate is ground into lime (\( \text{CaO} \)), used in cement, paper manufacturing, and water treatment. The formula \( \text{CaCO}_3 \) belies its multifaceted role—acting as both a feedstock and a pH regulator.
Carbonate’s influence reaches even the microscopic scale, particularly in biological calcification.
Marine organisms such as corals and mollusks precipitate calcium carbonate to construct hard structures. “The controlled deposition of \( \text{CaCO}_3 \) is a marvel of bioengineering,” notes Dr. Markova.
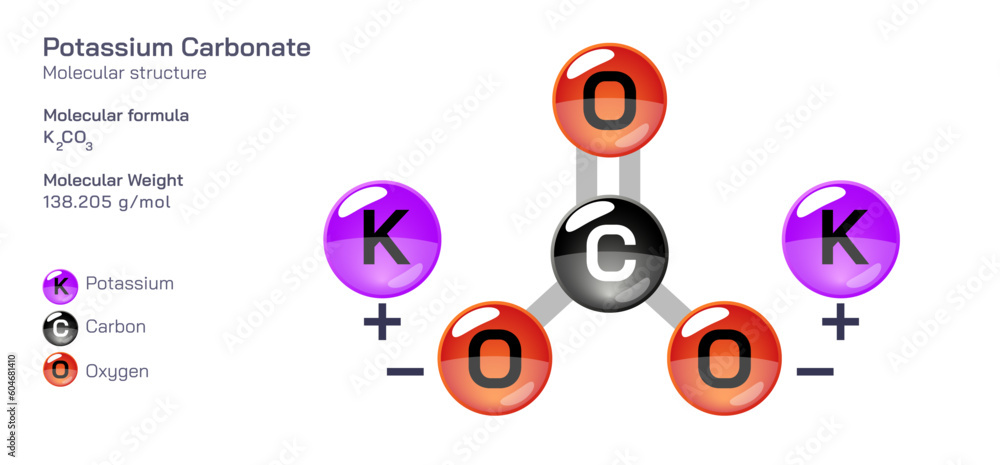
“These lifeforms regulate ion concentrations to form crystalline templates—tiny carbonate units bound in protein matrices—that yield materials stronger than most synthetic composites.” This natural mineralization process inspires innovations in biomimetic materials and carbon capture technologies, where synthetic carbonates could sequester atmospheric CO₂ at scale. Industrial applications further underscore carbonate’s versatility. In the pulp and paper industry, alkaline sodium carbonate (\( \text{Na}_2\text{CO}_3 \)) neutralizes acidic byproducts, prolonging fiber strength and improving whiteness.
Similarly, in fire suppression systems, sodium or potassium carbonates release non-toxic, alkaline extinguishing agents when triggered—rodeways of safety in commercial buildings.
The environmental impact of carbonate chemistry is profound. Oceanic carbonate systems absorb nearly a third of anthropogenic \( \text{CO}_2 \), mitigating climate change but triggering ocean acidification.
“As \( \text{CO}_2 \) levels rise, seawater pH drops, reducing carbonate ion availability,” warns oceanographer Dr. Rajiv Patel. “This disrupts shell-forming organisms and the entire marine food web—an urgent call for integrated carbon management.” Yet, this very reactivity is being harnessed: direct air capture technologies incorporate carbonate chemistry to convert airborne CO₂ into stable mineral forms, turning threat into opportunity.
From molecular geometry to global biogeochemical cycles, the formula \( \text{CO}_3^{2-} \) encapsulates a dynamic player in Earth’s chemistry. Its role spans natural processes—carbonate rock formation, ocean buffering, shell formation—and engineered innovations in construction, environmental remediation, and sustainable materials. “Carbonate chemistry is the silent architect,” says Dr.
Markova. “Its simplicity belies a complexity that underpins ecosystems, industry, and the planet’s long-term carbon balance.” As research advances, the formula \( \text{CO}_3^{2-} \) continues to illuminate pathways for scientific discovery and practical transformation.
Understanding the molecular formula of carbonate is not merely academic—it is essential for deciphering the chemistry that sustains life and enables human progress.
In a world increasingly shaped by carbon dynamics, mastering carbonate transforms theoretical insight into tangible solutions for environmental resilience and technological evolution.

Related Post
Mastering the Dv Lottery Photo Requirements: Your Ultimate Guide to Stand Out in 2024

Surrealestate Cast Meet the Stars of the Show in a Dreamweave of Talent and Spectacle

Typing Games: Sharpen Speed and Accuracy Like Never Before

Unlocking the Carapace: Nature’s Armor and Its Critical Role in Survival

