Unlocking Hydrogen Bromide’s Secrets: How Lewis Dot Structures Reveal Its Chemical Behavior
Unlocking Hydrogen Bromide’s Secrets: How Lewis Dot Structures Reveal Its Chemical Behavior
In the shadow of elemental periodicity, few compounds illustrate the elegance of molecular design as vividly as hydrogen bromide (HBr). A simple binuclear molecule composed of one hydrogen atom and one bromine atom, HBr defies complexity through the precision of its Lewis dot structure. Understanding this structure not only demystifies its geometry and bonding but also illuminates its role as a powerful acid, nucleophile, and reactant in industrial and laboratory chemistry.
By examining the arrangement of electrons in HBr’s Lewis dot structure, scientists and students alike gain critical insight into its polarity, stability, and reactivity—cornerstones of modern chemical understanding.
The Atomic Architecture of HBr: From Nucleus to Bonding
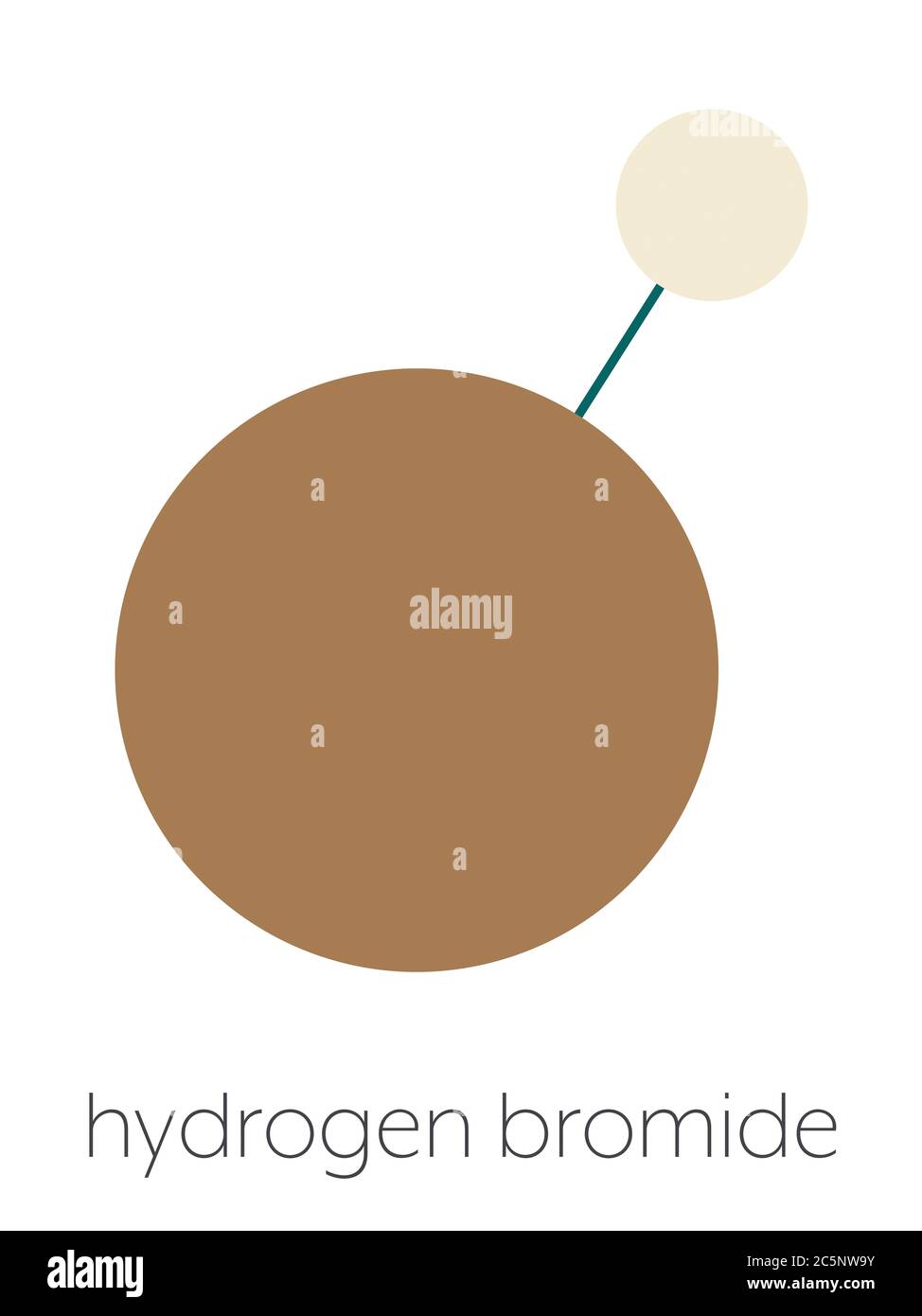
The Lewis dot structure for hydrogen bromide centers on the sharing of a single electron pair between hydrogen (H) and bromine (Br), forming a polar covalent bond. In this model, hydrogen contributes one electron to the shared pair, occupying its only valence shell with two electrons—fully satisfying the duet rule.Meanwhile, bromine, with seven valence electrons, provides one electron to complete the pairing, leaving six unshared electrons in its outermost shell—characteristic of a valence shell electron capacity of eight, or “an octet.” The result—a simple covalent bond—reflects hydrogen’s electronegativity (2.20) being only moderately higher than bromine’s (2.96), making the bond polarized with a partial negative charge on bromine and a partial positive charge on hydrogen. This polarity is pivotal: it transforms HBr from an inert molecule into a strong acid and reactive intermediate. The spatial arrangement, confirmed by VSEPR theory, places the bond at a near-180-degree angle, though slight distortion occurs due to lone pair repulsion on bromine, preserving a linear geometry in the molecule.
A precise representation shows H bonded to Br via a single arrow (::), with each atom displaying its valence electrons and no formal charge. The structure reveals minimal lone pairs on hydrogen (zero) and one lone pair on bromine—responsible for subtle molecular interactions and influencing reactivity patterns in reactions.
Electron Distribution and Chemical Reactivity Decoded
The Lewis dot structure lays the foundation for understanding HBr’s reactivity. Despite only one bond, the polar nature—evidenced by unequal electron sharing—drives its behavior in aqueous and organic media.Bromine’s higher electronegativity pulls electron density toward itself, generating a partial negative charge (δ⁻) on Br and a partial positive charge (δ⁺) on H. This polar character makes HBr a potent protic acid, readily donating the hydrogen ion (H⁺) when dissolved in water to form H₃O⁺ and Br⁻.
Moreover, the presence of a lone pair on bromine endows HBr with nucleophilic potential.
In organic synthesis, bromide ions serve as ideal leaving groups or reactive species in substitution and elimination reactions. The ability of Br to stabilize negative charge via expansion of its octet—possible due to access to d-orbitals—further enhances its versatility in reagents designed for halogenation and coupling processes.
Bond Energy, Stability, and Industrial Relevance
The covalent bond in HBr, while relatively weak compared to stronger bonds like C-C or O=O, balances stability with reactivity. Its bond dissociation energy hovers around 36 kcal/mol, enabling dissociation in dilute aqueous solutions without complete decomposition.This controlled instability suits applications ranging from flame retardants to pharmaceutical intermediates, where selective reactivity is essential. In industry, HBr stands as a cornerstone reagent. In petrochemical processing, it functions in alkylating agents for synthesizing olefins and silicones.
In analytical chemistry, standardized HBr solutions calibrate pH detectors and assess acid strength across compounds. Its role in educational labs remains irreplaceable: observing its rapid acid dissociation and formating hydrobromic acid in solution provides tactile insight into Br⁻ nucleophilicity and H⁺ acidity.
Safety and Handling: The Need for Precision
Despite its utility, HBr demands careful handling due to its corrosive and volatile nature.The Lewis structure, while chemically informative, underscores why excess HBr vapor irritates mucous membranes and corrodes surfaces. Laboratory protocols mandate fume hoods, protective gear, and sealed containers—measures rooted in the molecule’s polarity and reactivity. Understanding structure-guided behavior ensures safer, more effective use across settings.
From classroom demonstrations to industrial reactors, the Lewis dot structure of HBr emerges not just as a static diagram, but as a dynamic lens into molecular behavior. Its simple form belies a complex role shaped by electron distribution, polarity, and structural geometry—factors that define hydrogen bromide’s enduring significance in chemistry.
The Enduring Significance of Structure in Chemical Identity
In chemistry, structure is synonymous with function. The Lewis dot structure of HBr exemplifies how electron arrangement dictates molecular identity, reactivity, and application.From explaining acidity and nucleophilicity to guiding safe industrial practices, this molecular blueprint enables innovation across science and engineering. As research probes new materials and reactions, revisiting foundational structures like HBr’s remains essential—not just for education, but for advancing the frontiers of chemical science.



Related Post

Blue Lock Rivals: The Ultimate Showdown of Talent, Schemes, and Rivalries

From Fur Trades to Retail Powerhouses: The Enduring Legacy of Hudson’s Bay Company

Never Been Kissed Cast Would Redefine Behind-the-Scenes Storytelling Through Authentic Chemistry

TikTok Ban on iPhone: What You Need to Know Before It Reshapes Your Digital Life

