Unlocking General Chemistry 106: Sapling Learning Homework Answers That Actually Work
Unlocking General Chemistry 106: Sapling Learning Homework Answers That Actually Work
For students diving into General Chemistry 106, mastering foundational concepts is not optional—it’s essential. At Sapling Learning, homework answers for this course are engineered to transform abstract principles into clear, conquerable knowledge. These answers don’t just restate textbook definitions—they contextualize, apply, and reinforce understanding through strategic, evidence-based problem-solving.
In General Chemistry 106, where topics range from atomic structure to chemical equilibrium, comprehensive homework assistance becomes the bridge between confusion and confidence. What sets Sapling’s approach apart is its emphasis on clarity, precision, and real-world relevance—ensuring learners don’t merely solve equations but truly understand the “why” behind them.
At the core of Sapling Learning’s General Chemistry 106 homework support lies a systematic deconstruction of complex topics.
Students frequently struggle with abstract concepts like ionization energy trends or acid-base equilibria, where rote memorization fails and deep comprehension is required. Sapling’s answers break down problems into digestible components, guiding learners through step-by-step reasoning that emphasizes logic over rote recall. For instance, when addressing equilibrium constants, the platform connects scenarios involving Le Chatelier’s principle directly to calculative work, showing how shifts in concentration or pressure influence system balance.
This contextual linkage transforms abstract equations into dynamic representations of real chemical behavior.
Core Principles in General Chemistry 106 Demystified: The course demands fluency in several interrelated domains, each addressed with targeted homework support: - Atomic Structure & Periodicity: Understanding electron configurations, orbital shapes, and trends in atomic radius, electronegativity, and ionization energy relies on Sapling’s detailed explanations paired with guided practice. Students learn how quantum numbers dictate behavior and how periodic patterns emerge from electron arrangement—critical for predicting reactivity.
- Chemical Bonding: Sapling Learning emphasizes molecular geometry, hybridization, and bond polarity through both qualitative reasoning and quantitative models. Homework answers illustrate how VSEPR theory predicts shape and how electronegativity differences determine bond type—empowering students to visualize molecular structure from atomic details. - Thermochemistry and Reaction Energetics: Calculations involving enthalpy changes, Hess’s Law, and calorimetry pose frequent hurdles.
Sapling’s solutions present balanced equations, dielectric interpretations, and real-world examples—such as fuel combustion or phase transitions—helping students connect abstract thermodynamics to tangible energy flows. - Acid-Base Chemistry: From Brønsted-Lowry theory to buffer calculations, Sapling’s homework answers clarify misconceptions, showing how pH is governed by hydrogen ion concentration and how conjugate acid-base pairs stabilize solutions. Stepwise problem-solving demystifies titrations and equilibrium shifts.
- Equilibrium and Thermodynamic Favorability: Where chemical systems settle at balance, students encounter intricate calculations of Keq, Gibbs free energy, and reaction quotients. Sapling’s framed explanations turn complex inequality expressions into intuitive guides for predicting spontaneity.
One of Sapling Learning’s most impactful features is its diagnostic scaffolding—homework answers don’t just provide solutions but expose gaps in reasoning.
Each step is annotated to reveal underlying assumptions, common pitfalls (like sign errors in energy calculations), and alternative approaches. This transparency transforms errors into learning moments. For example, a misapplied phase-conversion rule in a thermochemistry problem might be flagged, with a clear correction that reinforces dimensional analysis.
Real-world applications anchor standing and new knowledge. Sapling Learning integrates chemistry into everyday contexts—analyzing soda fizz, explaining combustion efficiency, or interpreting blood pH—making abstract laws relevant and memorable. This relevance strengthens retention and sharpens analytical thinking.
Students using Sapling’s General Chemistry 106 homework solutions consistently report improved scores and deeper comprehension. The platform’s strength lies not in plug-and-chug shortcuts but in fostering genuine understanding through structured, reflective problem-solving. Scenario-based practice, error analysis, and progressive difficulty build both technical skill and critical thinking.
By turning homework into targeted coaching, Sapling transforms challenges into opportunities—proving that mastering chemistry is not about memorizing formulas, but about understanding the molecular story behind every reaction.
With Sapling Learning’s targeted homework guidance, General Chemistry 106 evolves from a daunting syllabus into a navigable intellectual journey—where each answer reinforces not just content, but capability.

Related Post

Hd Hub4u: Your Ultimate Destination for Streaming and Downloading Ultra-High-Definition Movies
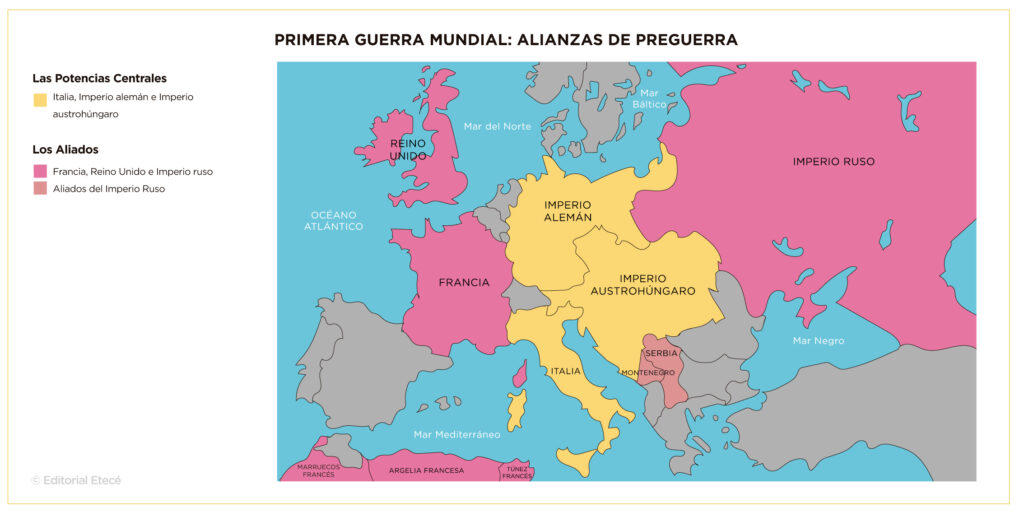
What Year Defines the Twentieth Century? Unlocking the Century’s Exact Boundary
Unlock the Power of Plantet Clicker: The Ultimate Tool for Sustainable Resource Farming

Digdid Io: Unlocking the Future of Intelligent Systems Through Adaptive Digital Identity

