The Powerful Chemistry Behind Household Staples: Vinegar and Baking Soda’s Reactive Revelation
The Powerful Chemistry Behind Household Staples: Vinegar and Baking Soda’s Reactive Revelation
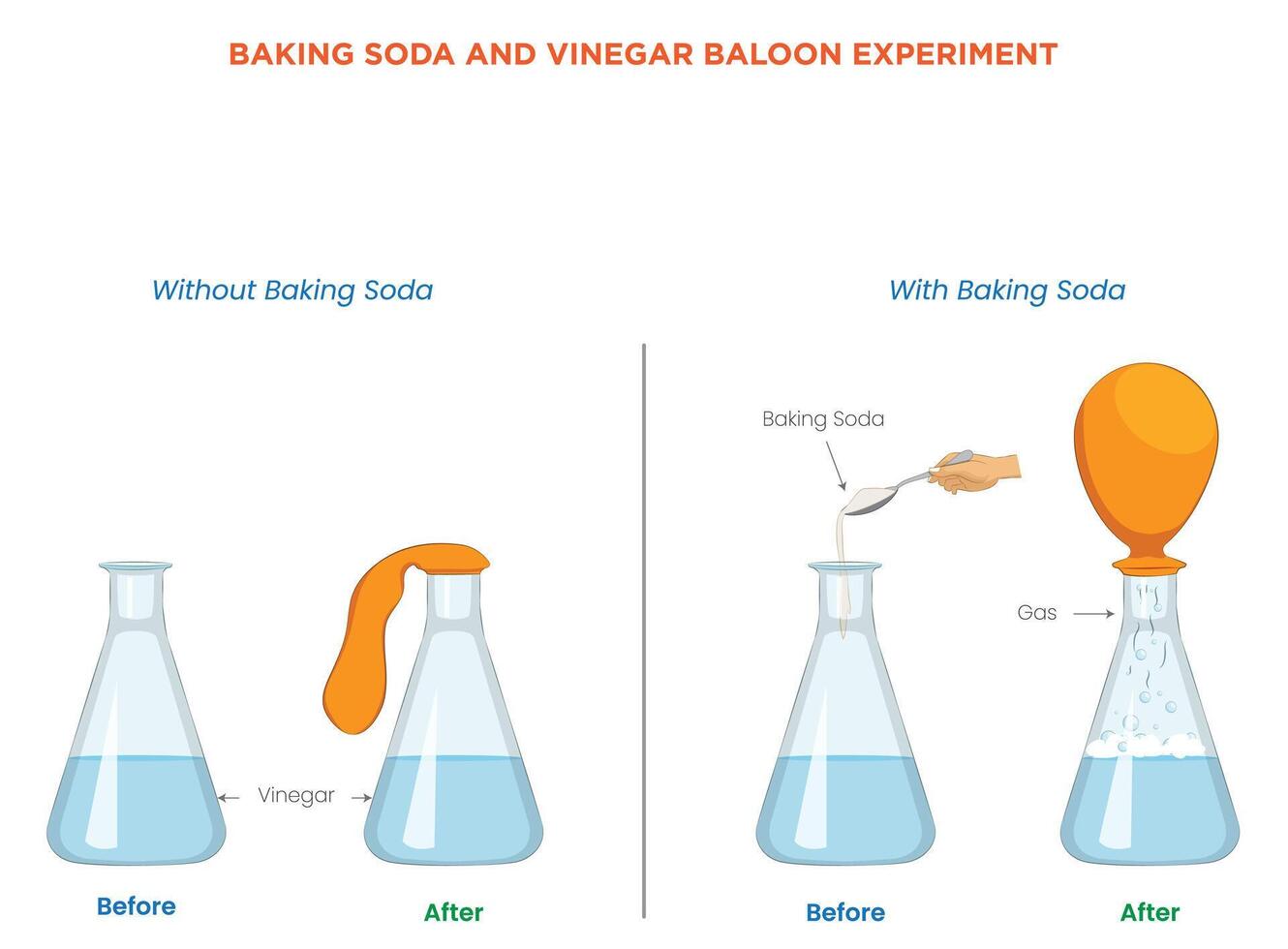
When vinegar meets baking soda, a cascade of visible and invisible chemistry erupts—bubbles, fizz, and a fizzy reaction that has captivated curious minds for generations. This simple, accessible interaction between acetic acid and sodium bicarbonate is far more than a kitchen party trick; it’s a textbook example of an acid-base reaction with real-world applications, educational value, and surprising utility. More than just a basis for homemade volcanoes or cleaning hacks, this dynamic duo illustrates fundamental chemical principles that inspire both scientists and everyday users alike.
Understanding the reaction begins with identifying the core reactants. Vinegar, a common household liquid, is primarily solution of acetic acid (CH₃COOH)—a weak but effective organic acid. Baking soda, technically sodium bicarbonate (NaHCO₃), serves as a mild base.
When these two substances combine—whether stirred, poured, or repurposed in controlled experiments—their chemical composition triggers a transformation.
The Molecular Dance: What Happens When Vinegar Meets Baking Soda?
At the molecular level, the interaction is a classic acid-base neutralization. The acetic acid molecules donate hydrogen ions (H⁺) to the bicarbonate ions (HCO₃⁻), resulting in the formation of carbonic acid (H₂CO₃), a transient but unstable compound.“Carbonic acid quickly decomposes into water and carbon dioxide gas,” explains chemists at the American Chemical Society. “This effervescence—bubbling and fizzing—is the visible signature of the reaction.” The reaction can be summarized by this equation: CH₃COOH + NaHCO₃ → CH₃COONa + H₂O + CO₂↑ Sodium acetate (NaCH₃COO) and carbon dioxide gas form the byproducts—salty water remains as a liquid residue—while the gas escapes as silent bubbles. The final mixture often feels cool to the touch, a physical sensation tied to energy changes during the chemical transformation.
Beyond this core equation, the reaction is exothermic—releasing just enough heat to raise the temperature slightly but not enough to cause danger under typical conditions. This subtle thermal output underscores why such reactions are both observable and safe when handled properly.
Though often associated with science demonstrations, the vinegar-and-baking-soda reaction serves multiple everyday functions.
Its fizzing action excels as a dependable cleaning agent, dissolving mineral deposits and breaking down grime through the acidic environment. In kitchens, it neutralizes odors and aids in deodorizing surfaces and drains, making it a go-to for natural, chemical-free maintenance.
Educators frequently leverage this reaction to teach foundational chemistry concepts—acids, bases, gas production, and reaction indicators. “It’s tangible,” notes education researcher Dr.
Elena Torres. “Students see bubbles on demand, witnessing abstract ideas like pH shifts and molecular reactivity instantly.” This hands-on utility elevates the reaction from novelty to necessity in STEM learning environments. Health and Safety Considerations are straightforward: both substances are non-toxic and generally safe when used correctly.
However, direct contact with undiluted vinegar may irritate sensitive skin or eyes, while excessive brushing with baking soda can stain teeth or sensitive mucous membranes. Always handle automatically in controlled doses—such as using two cups of baking soda per cup of vinegar—to maintain effective yet gentle reactions.
Beyond the Basics: Creative and Professional Applications
The versatility of this reaction extends beyond household cleaning and education.In restaurant kitchens, chefs use controlled acid-base bursts to deliquesce fats or lighten textures, particularly in delicate sauces. In industrial settings, similar neutralization principles assist in waste treatment, neutralizing acidic effluents using alkali solutions derived from such reactions. Moreover, the vinegar-and-baking-soda dynamic inspires DIY experimentation.
Makers and hobbyists use this pairing in home labs, crafting carbon dioxide through gas-sensitive sensors or crafting fizz-based cleaning products. Each application reflects a careful balance: maximizing the reaction’s vigor while ensuring safety and efficiency.
The spectacle and predictability of this reaction also make it a teaching tool for exploring variables in chemical systems.
Science educators routinely vary concentrations, ratios, and temperatures to illustrate how these factors influence reaction speed and gas output—illuminating core principles of chemical kinetics and equilibrium.
Reaction by Numbers: Key Data and Insights
The reaction proceeds with measurable efficiency. A typical household mixing ratio of one vinegar (5% acetic acid) to one tablespoon of baking soda generates approximately 750 mL of carbon dioxide gas—enough to lift a small balloon or inflate a soda bottle. The fizziness escalates rapidly at first, peaking in bubbles before tapering as reactants diminish.pH testing reveals the shift from acidic (vinegar, pH ~2.5) to near-neutral (baking soda solution, pH ~8–9), reflecting the acid’s neutralization. This pH transition is detectable even to casual observers using simple pH strips, reinforcing the reaction’s sensory immediacy. Scientific Clarifications Though frequently misunderstood, this reaction does not create new substances beyond the molecular byproducts—no combustion, no radiation, and no hazardous byproducts under normal use.
It remains a textbook example of a Class A acid-base reaction: rapid, observable, and gas-releasing. Safety and efficacy depend less on complexity than on understanding scale, ratios, and context.
The Broader Impact: Why This Reaction Endures as a Cultural and Scientific Touchstone
Vinegar and baking soda’s enduring appeal lies in their dual simplicity and depth.As household staples, they offer immediate utility—cleaning, odor control, specialized cooking—grounded in solid chemistry. As educational tools, they demystify abstract scientific concepts through tangible, repeated phenomena. Their visible transformation—bubbles, fizz, and cooling—makes invisible molecular interactions visible, bridging perception and understanding.
This reaction exemplifies how fundamental science, when connected to daily life, transcends the laboratory. It empowers users—from students to homeowners—to engage with chemistry confidently, safely, and with curiosity. Behind every bubble rises a lesson; behind every clean, a principle.
This simple pairing is not just a reaction—it’s a portal into the elegance of chemical transformation.
Final Thoughts: Embracing the Chemistry of Everyday Reactions
The vinegar-and-baking soda interaction stands as a testament to the invisible science woven through everyday existence. Far beyond a viral video or fleeting gag, it reveals how common substances, when combined with intention, enact visible change— bubbling, fizzing, and transforming.These reactions anchor curiosity, fuel discovery, and prove that chemistry is not confined to labs, but lives within the kitchens, classrooms, and homes we inhabit daily. In mastering these simple interactions, we unlock a deeper appreciation for the molecular choreography shaping our world—one fizz at a time.



Related Post

How Many Miles From Las Vegas to Reno? The Straight Grip on Distance

Broward County’s Digital Evolution: How SOC-as-a-Service is Transforming Public Safety in South Florida

Pacers Clobber Celtics in Packing Pace: Game 3 Box Score Reveals Defensive Fortitude and Offensive Surprises

Maligoshik A: The Unseen Architect of Oceanic Innovation

