The Periodic Table’s Metal-Nonmetal Divide: Elemental Forces Shaping Our World
The Periodic Table’s Metal-Nonmetal Divide: Elemental Forces Shaping Our World
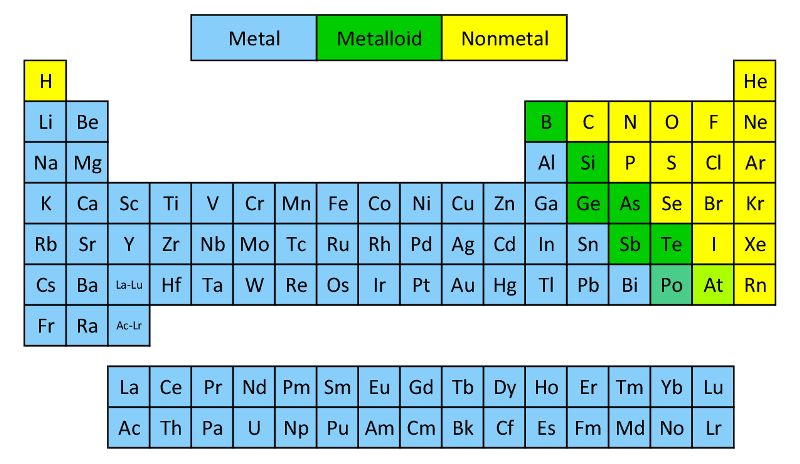
At the heart of chemistry lies a fundamental classification that governs the reactivity, structure, and utility of elements: metals and nonmetals, organized across the Periodic Table. This divide is more than a theoretical divide—it defines everything from the materials in our smartphones to the atmospheric gases that sustain life. Metals, broadly distributed across the left and center of the table, exhibit conductive properties, malleability, and a metallic luster, while nonmetals—clustered primarily in the upper right—display diverse behaviors ranging from brittle solids to gaseous forms, defining everything from molecular bonds to fuels.
This structural and functional contrast makes understanding their distribution and characteristics essential to science, industry, and everyday life.
Defining Metals: industrious Elements of Conductivity and Strength
Metals occupy the vast majority of the Periodic Table’s alkaline-earth and transition blocks, spanning groups 1 (alkali metals), 2 (alkaline earth metals), and 3–12 (transition metals), plus rare seconds in the lanthanide and actinide series. Characterized by their ability to conduct heat and electricity, metals readily lose electrons—forming positive ions—and fuse into ductile, lustrous materials.Their shares of the periodic chart’s mass and reactivity underscore their engineering importance—aluminum rare in jewelry but indispensable in aircraft, while iron forged the foundation of human civilization. - **Alkali Metals (Group 1):** The most reactive metals, including lithium, sodium, and potassium, with atomization energies so low they ignite violently in air. Sodium’s bright yellow flame and potassium’s lilac glow are iconic lab demonstrations.
“Sodium’s flammability isn’t just dramatic—it’s a red flag for reactivity,” explains chemical safety expert Dr. Elena Torres. - **Alkaline Earth Metals (Group 2):** Calcium, magnesium, and radium, less reactive than alkali metals but still prone to oxidation.
Calcium’s luminescence when shaved in air reveals a key diagnostic test. - **Transition Metals (Groups 3–12):** Iron, copper, chromium, and gold form the backbone of industrial chemistry. Their partially filled d-orbitals enable variable oxidation states, essential for catalysts in petroleum refining and batteries.
Copper’s resistance to corrosion powers electrical wiring; chromium’s alloying ability yields hard, wear-resistant steels. Metals also dominate the periodic layout’s structural logic. The s- and d-blocks entsembles—grouped vertically—highlight recurring trends in atomic radius, ionization energy, and electronegativity, guiding predictions of chemical behavior.
Defining Nonmetals: Diverse Elements of Reactivity and Complexity
Nonmetals resist metallic simplifications, instead showcasing a spectrum from gaseous diatomic molecules to brittle boron and conductive diamond. This group—encompassing halogens (Groups 17), noble gases (Group 18), chalcogens (Group 16), and pnictogens (Group 15)—reveals chemistry’s nuance: from energetic halogen exchange to inert persistence. Unlike metals, nonmetals tend to gain or share electrons, forming covalent bonds that define organic and inorganic compounds alike.- **Halogens (Group 17):** Fluorine, the most electronegative element, attacks almost any surface—even platinum—powering disinfectants and refrigerants. Chlorine’s oxidizing strength enables water treatment; iodine’s slow reaction with starch enables invisible moisture detection. - **Noble Gases (Group 18):** Helium, neon, argon, krypton, xenon, and radon occupy a unique position—monovalent or inert, these nonreactive gases stabilize high-tech applications, from neon sign lighting to cryogenic helium in MRI machines.
Though often colorless and odorless, xenon’s density makes it a strategic industrial gas. - **Chalcogens (Group 16):** Oxygen, sulfur, selenium, tellurium, and polonium drive biological and industrial cycles. Oxygen’s role in respiration and combustion is irreplaceable; sulfur, though toxic in excess, forms vital sulfides and fuels chemical manufacturing.
- **Pnictogens (Group 15):** Nitrogen, phosphorus, arsenic, and bismuth display complex behaviors. Nitrogen, despite chemical inertness in its diatomic form, fuels life via ammonia and recombinant synthesis; phosphorus, highly reactive in white form, underpins fertilizers and DNA. The spacing between metal and nonmetal zones—where metalloids like silicon and germanium blur the line—reveals a periodic gradient of reactivity.
Silicium, a quintessential metalloid, combines structural strength (silicon dioxide in glass) with semiconductor flexibility, forming the backbone of modern electronics.
Periodic Trends: The Visual Logic Behind the Divide
The periodic table’s layout is not arbitrary—it encodes periodic trends that clarify the metals/nonmetals split. As atomic number increases left-to-right across a period, ionization energy rises and electronegativity strengthens, turning metals into increasingly reactive post-board elements.Atomic radius shrinks



Related Post
Victory Road Emerald Map: The Hidden Blueprint of Strategic Deception in Competitive Games
Seriesonline Net

Aryna Sabalenka’s Coach Salary Revealed: How Much Does The Lithuanian Star Command On The Court

“And It’s Closed… Again: Inside the Mind we Fear in Five Nights at Freddy’s Lyrics

