The Molecular Switch: How Enzyme Active Sites Govern Life’s Catalyst
The Molecular Switch: How Enzyme Active Sites Govern Life’s Catalyst
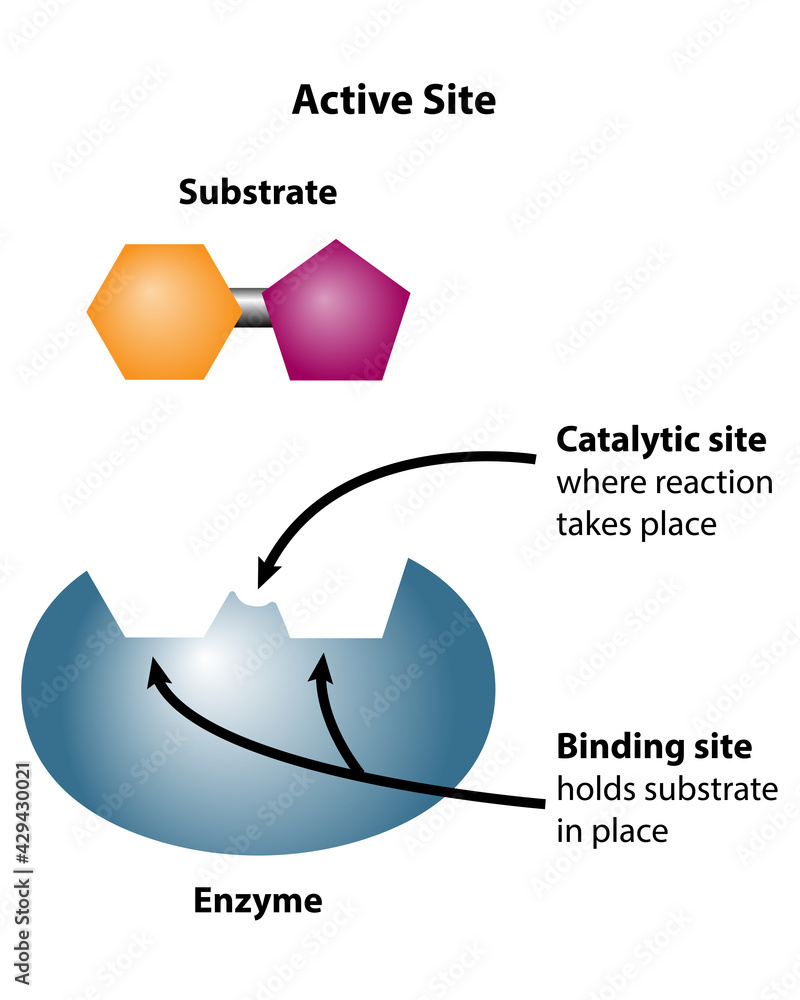
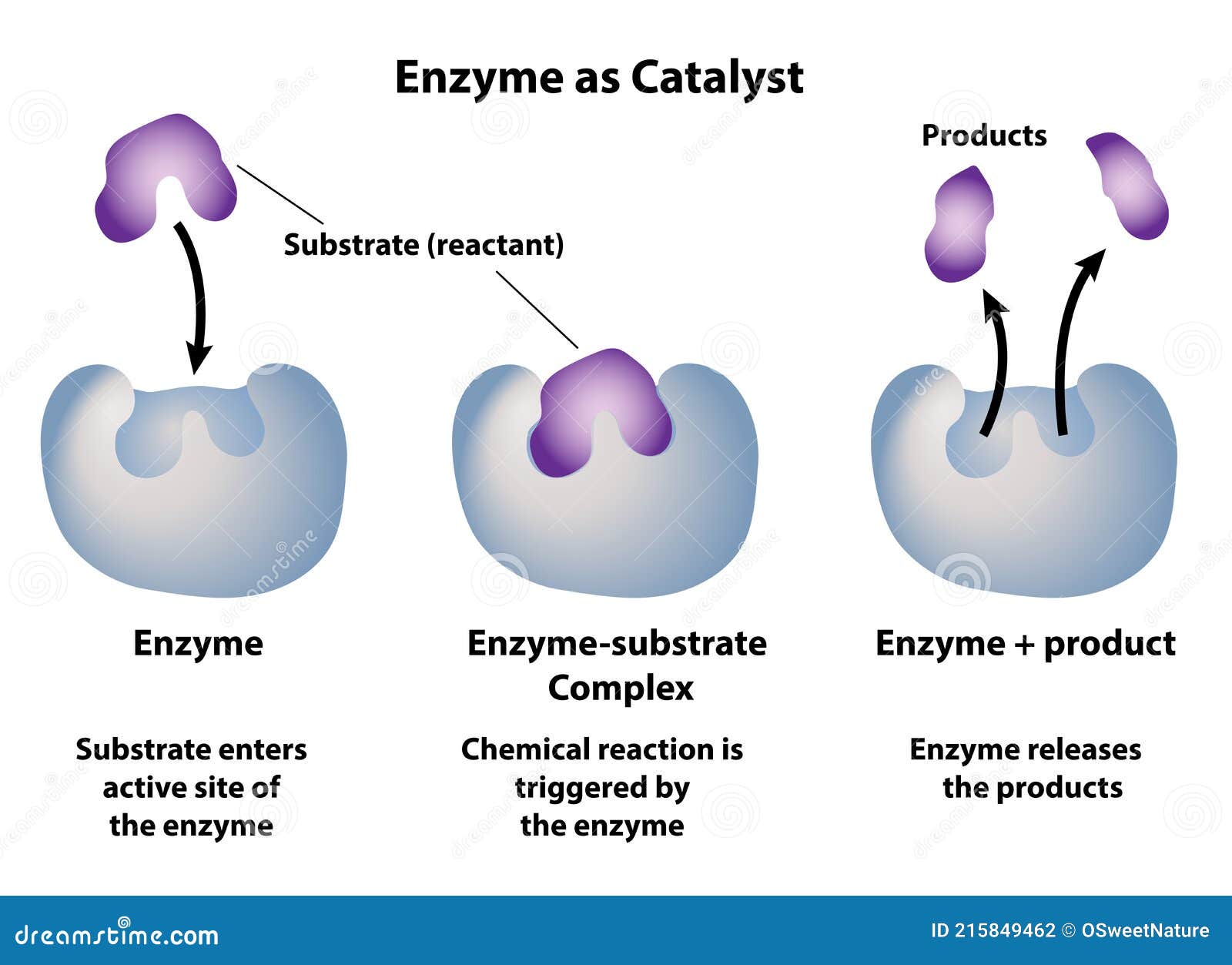
Enzymes are the silent architects of biological systems, orchestrating chemical reactions with precision that only the finest-tuned molecular machines can achieve. At the heart of their power lies the active site—the specific region where substrates bind and transformation occurs. This tiny, highly specialized pocket is the crucible of catalysis, enabling enzymes to accelerate reactions by factors of millions, without ever being consumed.
Understanding the active site reveals not just enzymology’s elegance, but its profound implications for medicine, biotechnology, and synthetic biology.
The Active Site: Nature’s Precision Engine
The active site is far more than a binding depression; it is a dynamically orchestrated pocket designed to stabilize transition states and orientation-dependent interactions between enzyme and substrate. Unlike generic binding regions, the active site is a three-dimensional microenvironment where amino acid residues deploy their unique chemical properties—hydrophobic pockets, charged ion clusters, hydrogen-bond networks, and metal ion cofactors—to orchestrate catalysis with atomic precision.Each enzyme’s active site is exquisitely complementary to its specific substrate, a fit so exact that even single amino acid substitutions can disrupt function. This specificity ensures metabolic efficiency and prevents unwanted side reactions. “The active site functions as a molecular lock,” explains Dr.
Elena Russo, a structural biochemist at the Max Planck Institute, “where only the correct key—substrate or cofactor—can trigger the transformation.” The active site’s power stems from multiple catalytic strategies: - Residue-mediated activation (acid-base catalysis) - Proximity and orientation effects that reduce reaction entropy - Metal ion coordination to stabilize negative charges - Proton shuttling via conserved amino acid side chains - Covalent intermediate formation for complex transformations This multifaceted role enables enzymes to achieve rate enhancements of up to 10¹⁷-fold compared to uncatalyzed reactions.
Structural Blueprint: The Engineering Marvel Behind Catalysis
The active site’s architecture is dictated by the precise spatial arrangement of amino acids encoded in the enzyme’s primary structure. These residues are positioned through intricate folding patterns—alpha helices, beta sheets, and loops—that form a constrained environment tailored for catalysis.The geometry of the active site ensures optimal alignment: substrates are held in precise positions, allowing reactive groups to react efficiently with minimal diffusion delay. This three-dimensional specificity is highlighted by the concept of the “induced fit,” a model describing how enzyme structures dynamically adapt upon substrate binding. “The active site isn’t rigid,” notes Dr.
Marcus Tran, a computational biologist specializing in enzyme dynamics, “it flexes and reshapes to better embrace its partner, enhancing catalytic efficiency by stabilizing the reactive transition state.” Beyond static architecture, the active site functions as a dynamic catalytic hub: - Hydrogen-bond networks lower activation energies by proton shuttling - Polar and charged residues modulate local dielectric constants - Flexible gatekeeping residues regulate substrate access and product release - Metal ions act as electrophilic catalysts or charge-relays in metalloenzymes This synergy between structure and function underscores why enzyme active sites remain among nature’s most elegant solutions to chemical challenge.
Enzyme Diversity: Active Sites Tailored to Life’s Demands
Across the 3.5 million known enzymes, active site designs span a spectrum of functional specializations—from oxygen-sensitive monooxygenases to metal-dependent oxidoreductases. Each active site reflects evolutionary optimization for a particular metabolic sector.Hepatic cytochrome P450 enzymes, for example, feature a heme-bound active site that enables precise hydroxylation of xenobiotics, while ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) recovers CO₂ in photosynthesis via a unique Mg²⁺ channel tailored to its dual carboxylation activity. This diversity ensures metabolic resilience: - Hydrolases cleave ester, glycosidic, or peptide bonds with sub-nanosecond turnover - Transferases manage group shuffling, with active sites hosting coenzymes essential for reaction coupling - Isomerases guide substrates through rearrangements, minimizing diffusion losses - Ligases couple reactions to ATP hydrolysis, enabling biosynthetic complexity Enzymatic specialization through active site innovation allows organisms to exploit nearly every conceivable chemical niche, illustrating how subtle environmental pressures shape molecular evolution.
Applications: Harnessing the Active Site for Innovation
Understanding enzyme active sites has catalyzed breakthroughs across science and industry.In biocatalysis, engineered active sites enhance substrate specificity and stability, enabling greener chemical synthesis that reduces toxic waste. Therapeutic development targets active sites to develop inhibitors—such as HIV protease inhibitors, designed to block substrate-frabMAX site binding—marking a cornerstone of modern drug design. Synthetic biology leans on active site knowledge to design artificial enzymes (`designors`) capable of non-natural transformations, expanding the biochemical toolbox.
Directed evolution platforms iteratively refine active site residues, accelerating enzyme performance for applications from biofuel production to environmental remediation. “The active site is our blueprint,” says Dr. Fatima Al-Sayed, a synthetic enzymologist at Stanford, “by decoding and reengineering it, we reprogram biological systems with unprecedented precision.” Looking forward, precision targeting of active sites promises sustainable solutions—from carbon-c Capturing enzymes to life-saving pharmaceuticals—turning the enzyme’s catalytic prowess into scalable, eco-friendly technology.
Active Site: The Keystone of Biochemical Control
Far more than a binding region, the enzyme’s active site is the epicenter of biological catalysis—where molecular architecture meets dynamic reactivity in a dance honed by evolution. Its three-dimensional perfection, chemical diversity, and adaptive flexibility make it one of nature’s most sophisticated catalytic systems. From medicine’s smallest drug molecules to biotech’s largest industrial enzymes, the active site remains the defining feature of enzymatic function.As research deepens our grasp, so too does our capacity to harness its power—ushering in a new era of innovation rooted in the microscopic marvel of life’s molecular machinery.

Related Post

What Making the World Live in Crowded Cities Defines a Dense Population — and Why It Shapes Our Future

Adam Sandler’s Military Roots: How a Serviceman’s Family Legacy Shaped a Hollywood Icon

What The Highest Paying Sport Reveals About Money, Skill, and Global Ambition
Play Anywhere for Free: Download Free Apps and Games on gens Spiel Onlin

