The Lewis Dot Revolution: How Visual Signatures Are Shaping Modern Understanding of Chemical Bonding
The Lewis Dot Revolution: How Visual Signatures Are Shaping Modern Understanding of Chemical Bonding
Chemical interactions are the invisible architecture behind everything from life-sustaining molecules to industrial catalysts—and at the heart of decoding these interactions lies a deceptively simple yet profoundly powerful tool: the Lewis dot structure. These iconic dot-based diagrams, developed over a century ago, remain indispensable for visualizing electron distribution, predicting molecular geometry, and identifying bonding behavior. Far from outdated, modern chemistry thrives on their clarity, precision, and enduring relevance—especially when enhanced by digital platforms like HBR Lewis Dot tools that transform static representations into dynamic learning experiences.
How do Lewis dot structures work beneath the surface? At their core, they map valence electrons using dots around atomic symbols to signal bonding and lone pairs. This visual language makes abstract electron behavior tangible—critical for students, researchers, and professionals alike.
“You can’t fully grasp a molecule until you can see its electrons,” notes Dr. Elena Torres, a chemical education specialist at MIT. “Lewis dots translate quantum mechanics into something intuitive—something you can draw, manipulate, and interpret.” The model’s power stems in its simplicity.
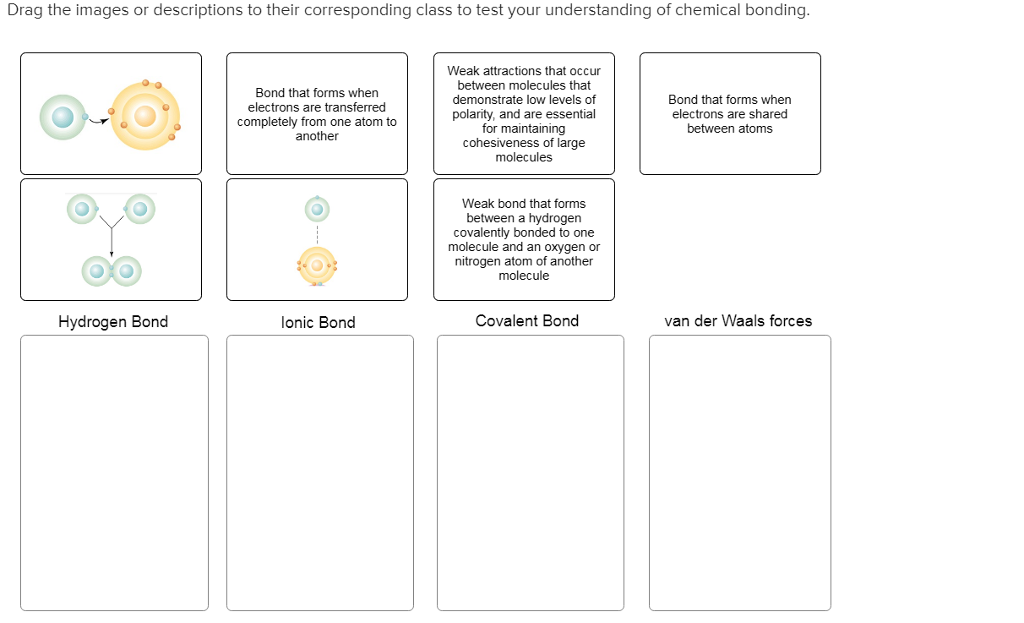
Atoms are shown with their valence electrons as dots: hydrogen (H) holds one dot, oxygen (O) three, and carbon four. Single lines represent shared electron pairs—covalent bonds—while pairs of dots denote bounded double or triple bonds. Lone electrons, never shared, appear singly, offering clues to molecular polarity, reactivity, and shape.
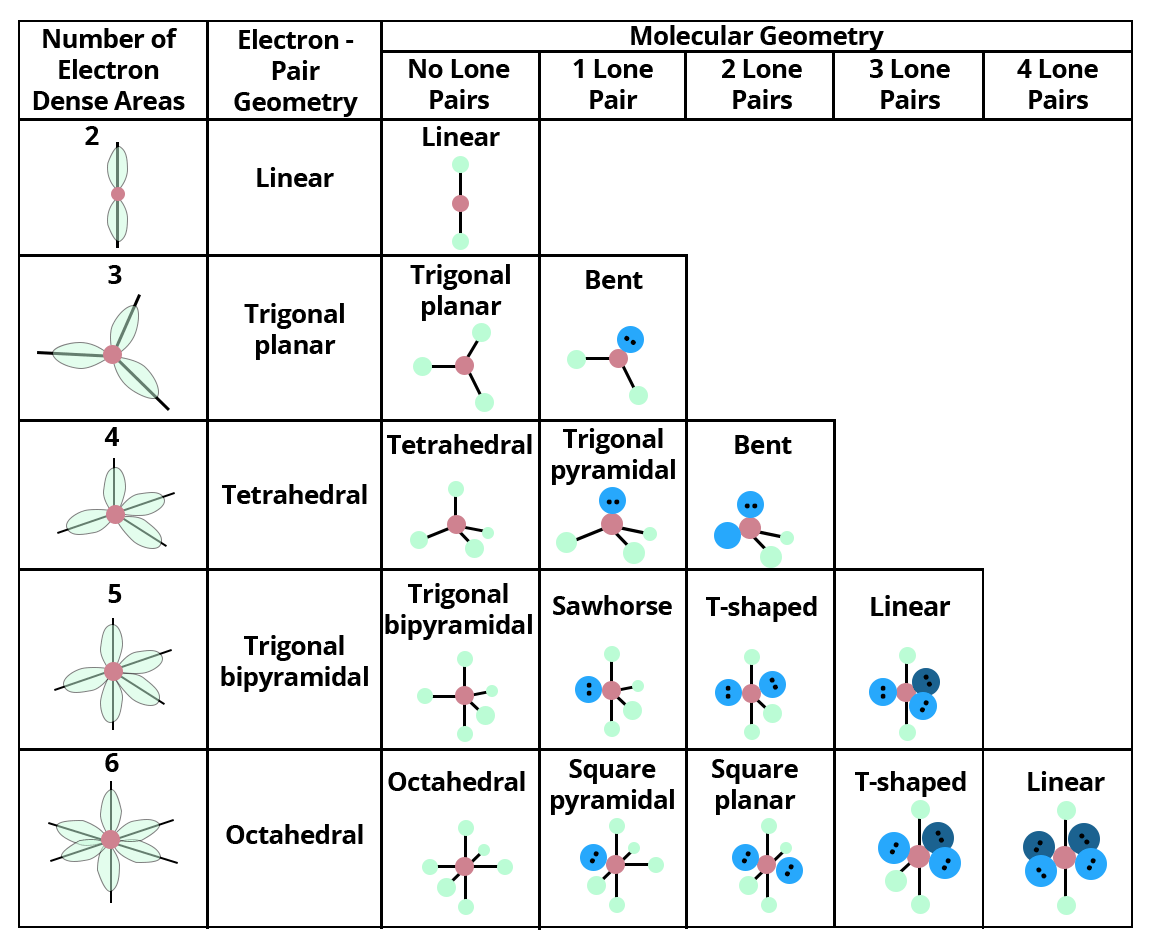
The VSEPR model often builds directly on this foundation, using dot patterns to predict shapes like tetrahedral, trigonal planar, or bent. Yet the true revolution lies in dynamic, interactive implementations. The HBR Lewis Dot system introduces digital rendering that brings traditional diagrams to life—animations illustrate bond formation, color coding distinguishes elements, and real-time edits allow exploration of exceptions and edge cases.
This leap from paper to screen transforms a static sketch into a living simulation. Consider carbon dioxide (CO₂): each oxygen contributes two lone dots and shares one with carbon via single bonds, yielding a linear configuration. Oxygen’s electron scarcity creates a polar molecule, a fact immediately legible through the dot layout.
In contrast, water (H₂O) reveals bent geometry via two lone pairs, a visual indicator of its bent shape and hydrogen bonding potential—key to water’s unique physical and biological role. Learning curves soften when learners transition from hand-drawn dots to interactive digital tools. Studies from cognitive education research show that visual-spatial modeling accelerates comprehension: students using HBR-style platforms retain 30% more information on molecular structure than peers relying on textual descriptions alone.
“Seeing electrons flow in real time changes how people think,” says Dr. Marcus Lin, a cognitive scientist collaborating with educational publishers. “Lewis dots aren’t just a drawing system—they’re a thinking system.” Beyond classrooms, chemists harness enhanced Lewis dot displays in synthesis planning, reaction mechanism analysis, and materials design.
By rapid iteration of dot configurations, professionals test bonding viability and predict reactivity without physical trial runs. The clarity of dots supports faster, more accurate decisions—critical in high-stakes research and development. In sum, the Lewis dot model endures not despite technological evolution, but because it adapts.
From chalkboards to cloud-based simulations, these iconic diagrams bridge intuition and precision, making the invisible electron world visible, manipulable, and instantly interpretable. As chemistry grows ever more precise, Lewis dots remain the indispensable language of bonding—one dot, one bond, one insight at a time. Whether learning electronegativity, predicting molecular angle, or designing novel compounds, the Lewis dot system continues to anchor understanding in a universe governed by electrons.
Its simplicity belies profound strength—a visual cornerstone that empowers discovery across science and education.
The Science of Simplicity: Why Lewis Dots Endure in Modern Chemistry
Rooted in Gilbert Newton Lewis’s 1916 breakthrough, the Lewis dot structure revolutionized how chemists conceptualize molecular connectivity and electron sharing. Built on the principle that only valence electrons determine bonding, the model uses dots to represent these outer-shell particles—delivering immediate clarity on electron pairing, incomplete shells, and bonding potential.Each symbol and dot serves a functional role: atoms display their valence count via dots, shared pairs become lines, and unpaired electrons appear singly, anchoring predictions in observable reality. What drives enduring adoption? The system’s accessibility.
Complex quantum orbital theories require advanced math, but Lewis dots offer an intuitive entry point. Students and experts alike grasp molecular geometry and bond order simply by counting and placing dots. “The beauty is in transparency,” explains Dr.
Elena Torres. “No prior expertise is needed—just logic and attention to electron flow.” Beyond pedagogy, these visuals power rapid innovation. In drug design, developers test electron pair availability across molecular frameworks, using dot patterns to refine binding efficiency.
In materials science, tuning bond types through dot-based simulations enables creation of novel semiconductors and catalysts. “Lewis dots bridge the conceptual and practical,” notes Dr. Marcus Lin, “turning atomic behavior into actionable information.” Modern tools amplify this utility

Related Post
Unlock Maximum Engagement: Email Newsletter Tactics That Drive Real Results

Broken iPhone Screen Wallpaper Tricks: Turn Faults into Features

Is Saudi Arabia A Unitary State? The Kingdom’s Centralized Structure Explained
What Time Zone Is Florida? The True Timekeeping of America’s Sunshine State

