The Chemical Powerhouse: Unlocking Water’s Secrets Through Oxidation and Reduction Potential
The Chemical Powerhouse: Unlocking Water’s Secrets Through Oxidation and Reduction Potential
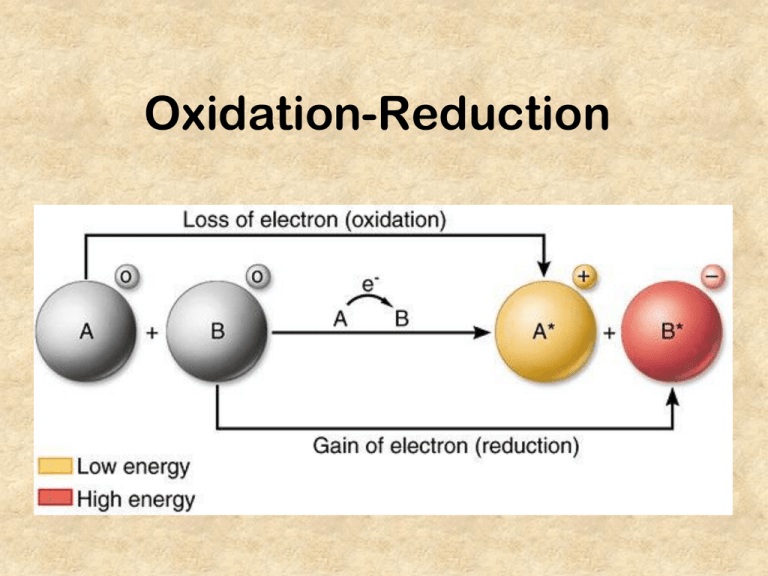
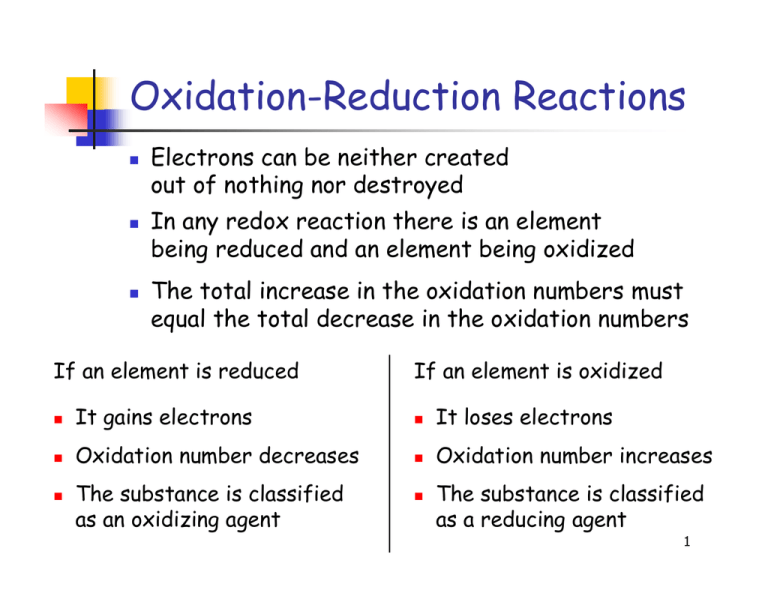
At the heart of countless natural and engineered chemical processes lies the fundamental dance between oxidation and reduction—electron transfers that govern everything from corrosion prevention to microbial metabolism. Central to predicting and controlling these reactions is the Oxidation-Reduction Potential (ORP) table, a scientific benchmark providing critical insights into the thermodynamic tendencies of chemical species in solution. By decoding ORP values, scientists and engineers determine whether a substance will act as an electron donor (reducing agent) or acceptor (oxidizing agent), guiding applications in environmental remediation, water treatment, industrial processes, and energy systems.
This article examines how the ORP table functions as a predictive roadmap, revealing oxidation and reduction dynamics with precision and clarity.
What Drives Electron Transfer? The Foundation of Reduction Potential
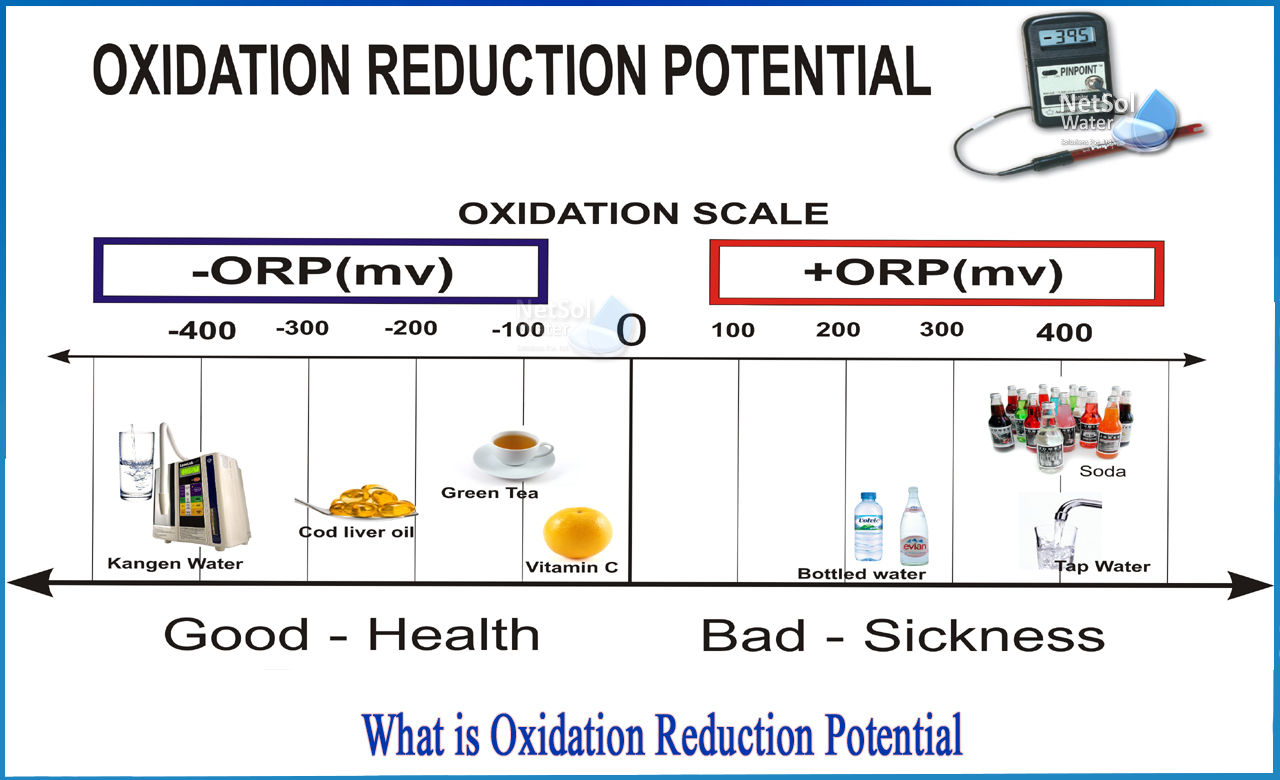
Reduction potential, expressed in volts, quantifies a species’ ability to gain electrons and undergo reduction.Putting it simply, the higher the ORP value, the stronger a substance’s electron-accepting power—its drive to “snatch” electrons from another molecule. Conversely, species with low or negative ORP values readily donate electrons and are strong reductants. The Oxidation-Reduction Potential Table compiles standard electrode potentials measured under controlled conditions—typically at 25°C, pH 7, and 1 molal concentration—for reference materials such as O₂/H₂O, Fe³⁺/Fe²⁺, and Cl₂/Cl⁻.
These values form a relative scale where: - Positive OFP (Open Circuit Potential) indicates oxidizing agents - Negative OFP values signify reducing agents According to Dr. Elena Marquez, environmental chemist at the National Center for Water Sustainability, “The ORP table isn’t just a list—it’s a dynamic guide that reveals thermodynamic feasibility. It tells us which redox reactions will spontaneously occur in a given solution environment.”
Understanding the chemical language of redox beginnt with key reference pairs.
The standard hydrogen electrode (SHE), assigned a potential of 0 V at all conditions, anchors the scale. At pH 0, O₂/H₂O holds +1.23 V, confirming its role as a powerful oxidizer. Meanwhile, Fe²⁺/Fe³⁺, commonly at –0.77 V, readily enables electron transfer in biological and geochemical systems.
The spread between positive and negative potentials underscores the vast redox landscape, with bacteria, metals, and organic compounds occupying critical mid-range zones.
Mapping Redox Behavior: A Breakdown of the Oxidation-Reduction Potential Table
The ORP table organizes electroactive species by their reduction tendencies, offering a predictive tool for complex chemical equilibria. A table’s value typically reflects reduction under standard conditions; real-world applications require adjustments for pH, ionic strength, and temperature—parameters accounted for via the Nernst equation. Common reference species and their standard potentials (versus SHE): - O₂ + 4H⁺ + 4e⁻ → 2H₂O → +1.23 V - Fe³⁺ + e⁻ → Fe²⁺ → +0.77 V - Cl₂ + 2e⁻ → 2Cl⁻ → +1.36 V - S⁶⁺ + 6H⁺ + 6e⁻ → S²⁻ → –0.22 V These values illuminate how different species interact: oxidizing Fe²⁺ requires a stronger oxidant (like O₂), while chlorine oxidizes iodide readily due to chlorine’s high ORP.“Each pair tells a story of reactivity,” notes Dr. Rajiv Patel, a physical chemist at MIT. “The table enables us to anticipate outcomes before experimentation—saving time, resources, and advancing innovation.”
The table also reveals redox shifts essential in biological systems.
For instance, cytochromes shuttle electrons using heme groups with specific heme potentials (often +100 mV to –300 mV), enabling energy conversion in cellular respiration. Here, small differences in oxidation potential govern electron flow, driving ATP synthesis.
Applications Across Industries: From Water Purification to Energy Storage
One of the most impactful uses of ORP tables lies in environmental and water treatment technologies. Industrial and municipal wastewater systems rely on ORP measurements to optimize chemical dosing—for example, using chlorine (ODP ~1.36 V) to Elsemette the disinfection process—while activated carbon filtration monitored by ORP ensures removal of organic contaminants through redox reactions.In corrosion science, ORP data guides cathodic protection strategies. Metals like iron corrode when their surface ORP drops below a depolarization threshold, allowing electrons to flow from an impressed current. Setting the right potentials using sacrificial anodes prevents structural degradation.
Emerging technologies such as iron-based rechargeable metal-air batteries exploit controlled redox chemistry. The ORP chart helps select cathode materials with ideal reduction potentials—ensuring efficient charging and discharging. In hydrogen fuel systems, proton-exchange membrane electrodes depend on stable ORP values to sustain electrolysis and fuel cell reactions.
Furthermore, in soil remediation, ORP monitoring evaluates biogeochemical conditions affecting microbial activity. Reductive dechlorination of pollutants like PCBs relies on microbes exploiting electron donors—processes guided by environmental redox potentials measured against the ORP table.
The Future of Redox Analysis: Precision and Predictive Power
Advances in sensor technology now enable real-time, in-situ ORP monitoring across diverse environments—water bodies, industrial reactors, and living tissues.Portable ORP meters paired with data analytics offer unprecedented control and feedback loops. As industries shift toward sustainable processes, understanding oxidation and reduction thresholds becomes critical to minimizing waste and maximizing efficiency. “The Oxidation-Reduction Potential Table remains an indispensable tool,” states Dr.
Marquez. “It bridges theory and application, allowing scientists and engineers to harness redox chemistry with precision. Whether treating water, storing energy, or probing life at the molecular level, mastery of ORP empowers innovation grounded in fundamental science.”
As research deepens into nanotechnology, synthetic biology, and climate-relevant redox cycles, the ORP table continues to evolve as a foundation—turning abstract electron exchanges into actionable knowledge, driving progress across science and engineering.
Mastering Electron Flow: Why ORP Matters Now More Than Ever
At its core, oxidation and reduction potential quantify the invisible flow of electrons—governing stability, reactivity, and transformation.The Oxidation-Reduction Potential Table distills this invisible world into comprehensible data, empowering infrastructure, sustainability, and discovery. From municipal water treatment to cutting-edge battery technology, understanding redox chemistry via ORP values is no longer optional—it’s essential. As we confront pressing environmental and energy challenges, leveraging this fundamental chemical insight ensures smarter, cleaner, and more resilient systems.
In the silent language of electrons, the ORP table speaks volumes.

Related Post

Decoding Carbon’s Cosmic Power: The Atomic Dance Revealed in Lewis Dot Structure

How Many Books Has Stephen King Written? A Definitive Count and Legacy in Literary History

Oswaldo Sánchez: Chile’s Quiet Catalyst for Social Change Through Urban Innovation

Stream East: The Hidden Lifeline Reshaping Urban Rivers and Global Water Futures

