Reactants of Electron Transport Chain: The Vital Fuel Powersing Cellular Energy
Reactants of Electron Transport Chain: The Vital Fuel Powersing Cellular Energy
Limitless biological power unfolds within the microscopic corridors of eukaryotic mitochondria, where the electron transport chain (ETC) operates as the engine of aerobic life. This chain, embedded in the inner mitochondrial membrane, transforms chemical energy into usable ATP through a meticulously choreographed sequence of redox reactions—each driven by key reactants that act as the chain’s essential fuel. Without these specific molecules, the ETC would stall, halting ATP production and threatening cellular function.
Understanding the reactants—electrons’ initial donors and electron acceptors—reveals not just the biochemical mechanics of energy conversion but also the fragile balance sustaining life at the molecular level.
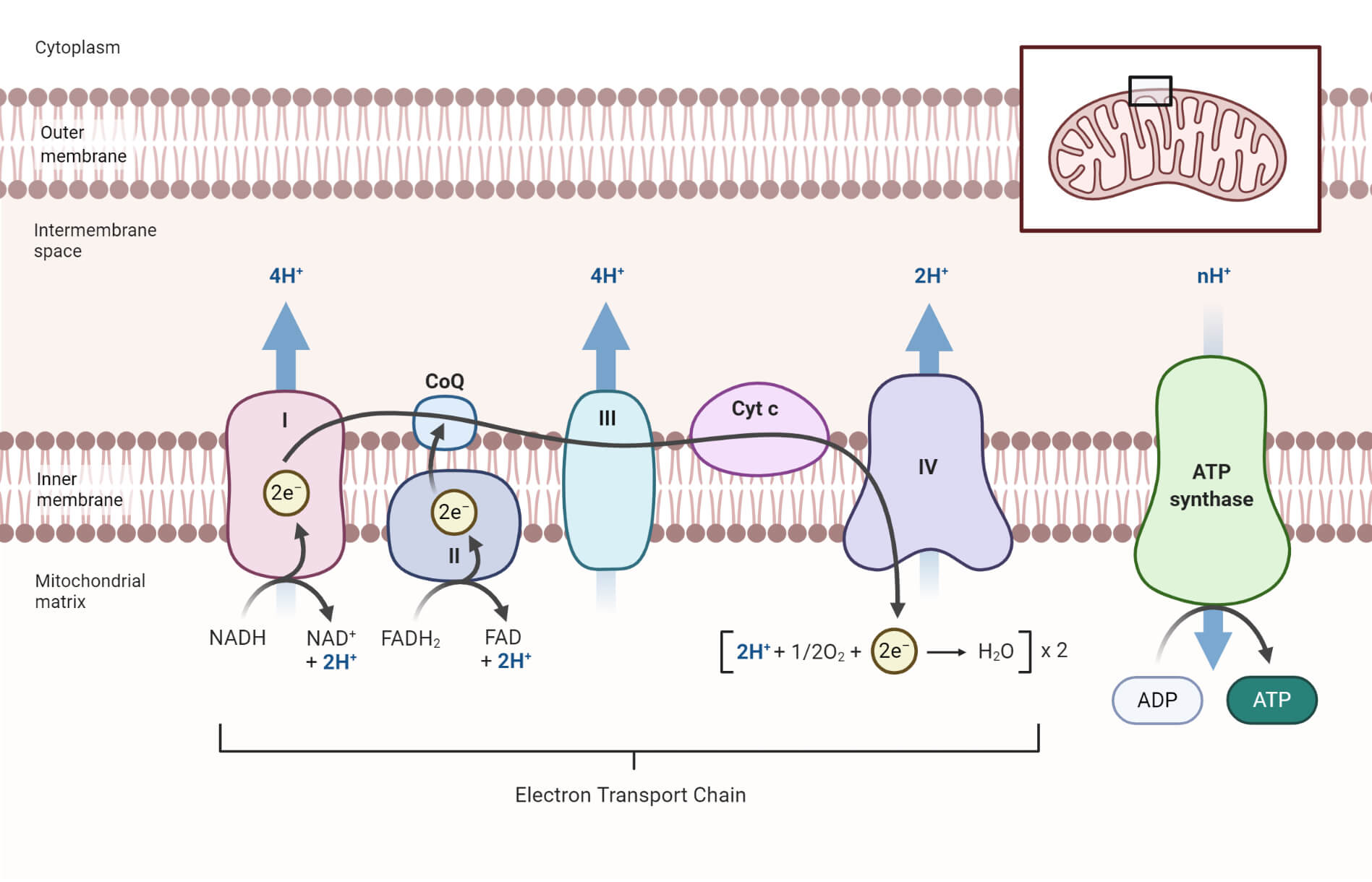
The primary reactants of the electron transport chain fall into two critical categories: electron donors that supply the electrons initiating the cascade, and molecular oxygen, the ultimate electron acceptor that ensures the process never ends. The ETC’s operation begins with tightly bound coenzymes and metal-based prosthetic groups that capture high-energy electrons, followed by a sequence of protein complexes—each dependent on precise reactants to drive proton pumping and maintain the electrochemical gradient.(Krebs, B., & Stryer, L.
*Biochemistry*, 7th ed.)
The Core Electron Donors:NADH and FADH₂
Central to the electron transport chain are NADH and FADH₂, two small coenzymes loaded with high-energy electrons derived from earlier metabolic pathways. NADH enters the ETC at Complex I, donating electrons that set the initial spark for electron flow. FADH₂, generated in the citric acid cycle, delivers electrons earlier in the chain—specifically to Complex II—bypassing an entry point but still feeding into the energy-generating process with moderate bioenergetic yield.
“NADH is the more potent electron donor, feeding into Complex I, while FADH₂ feeds at Complex II, yielding fewer protons per electron pair,” explains mitochondrial biologist Dr. Elena Petrova, underscoring the efficiency differences between these fuel molecules.(Petrova, E. *Mitochondrial Metabolism: Electron Flow and Cellular Energy*, 2022)
- NADH originates primarily from glycolysis (in cytoplasm, then fabs into mitochondria), the citric acid cycle, and fatty acid oxidation.
- FADH₂ arises exclusively during the citric acid cycle and beta-oxidation of lipids.
- Each molecule donates electrons at distinct points in the ETC, dictating the gradient strength and ATP output.
The Ultimate Electron Acceptor: Molecular Oxygen
No electron transport chain can function without molecular oxygen—O₂—acting as the terminal electron acceptor.
This gaseous molecule binds at the end of Complex IV (cytochrome c oxidase), where it captures electrons and protons to form water in a critical step: “Oxygen is indispensable; without it, electrons stall, protons flood back, and ATP production grinds to a halt,” notes Dr. Petrova. The reduction of O₂ to H₂O provides the thermodynamic push that sustains the proton gradient across the inner mitochondrial membrane, enabling ATP synthase to churn out energy at a rate sufficient to power cellular processes.
< requirements>(Department of Molecular Biology, Harvard Medical School, 2023)The stoichiometry of this final reduction is vital: each O₂ molecule binds four electrons and insects six protons, yielding two molecules of water and releasing energy that drives proton translocation.
This oxygen-dependent termination prevents electron leakage—linked to damaging reactive oxygen species—and ensures the ETC maintains both efficiency and safety.
The Role of Proton Gradients and Redox Potential
While electron donors and acceptors set the stage, the gradient of protons across the inner mitochondrial membrane constitutes the real workhorse of ATP synthesis. Complexes I, III, and IV shuttle protons from the matrix to the intermembrane space, generating a proton-motive force. The energy stored in this gradient powers ATP synthase, which uses the flow of protons to catalyze ADP and inorganic phosphate into ATP—a process remarkably efficient when reactants are fully utilized.
The redox potential of each electron carrier—NADH, ubiquinone, cytochrome c—also determines the direction and energy yield, illustrating how molecular reactivity defines bioenergetic efficiency.
Moreover, this system is finely tuned: fluctuations in NADH:FADH₂ ratios, oxygen availability, or inhibitor presence (such as lifestyle or disease factors) directly impact electron flow. Understanding these dynamics not only explains normal cellular respiration but also informs therapeutic targets for metabolic disorders, aging, and neurodegenerative conditions tied to impaired mitochondrial function.
From Molecular Fuel to Life’s Energy Currency
The reactants of the electron transport chain—NADH, FADH ₂, and oxygen—are not merely biochemical triggers; they are the biological linchpins transforming nutrients into usable energy. This intricate dance of electrons, protons, and oxygen powers every physiological process, from nerve impulse transmission to muscle contraction.
Without the precise supply and demand of these reactants, life as we know it would collapse within seconds. The ETC, fueled by carefully orchestrated electron donors and terminated by oxygen’s final grip, stands as a testament to nature’s elegance—where chemistry and biology converge to power existence at the cellular level.
In summary, reactants of the electron transport chain—NADH, FADH ₂, and molecular oxygen—form the biochemical foundation of aerobic respiration. Their study illuminates not only the engine room of the cell but also offers critical insights into health, disease, and the fundamental principles governing life’s energy economy.


Related Post

Is Rochester, NH the Hometown You’ve Always Wanted? A Deep Dive Into the Community, Culture, and Connection

How To Execute The Joe Lewis Shuffle: The Timeless Footwork That Defined A Generation of Boxing

Itchy Foot Meaning: Decoding the Subtle Language of Unease

Sabrina the Teenage Witch: A Magical Dive That Transforms Mushroom Town One Spell at a Time

