Master How to Find Percent Yield: Precision in Laboratory Values with Confidence
Master How to Find Percent Yield: Precision in Laboratory Values with Confidence
Understanding percent yield is essential for students, researchers, and chemists measuring reaction efficiency in the lab. More than a mere calculation, percent yield reveals the practical success of chemical syntheses, directly impacting research validity and industrial process optimization. Whether you're preparing a reaction or analyzing results, knowing how to determine percent yield accurately ensures reliable data and informed decision-making.
This guide distills the process into clear, actionable steps—from raw reaction outputs to final adjustments—with examples and practical tips to ensure mastery.
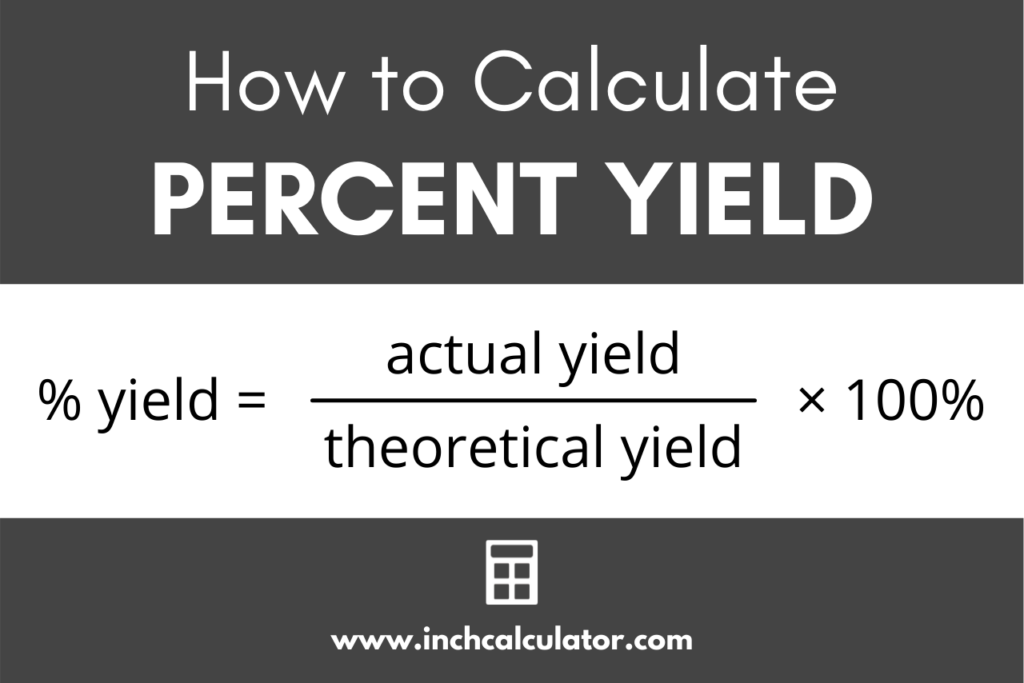
At its core, percent yield quantifies how much actual product was obtained compared to the theoretical amount predicted by stoichiometry. The formula is deceptively simple: (actual yield ÷ theoretical yield) × 100.
Yet, precision demands attention to detail—especially in calculating actual yield, which depends on accurate measurement and proper unit conversion. The theoretical yield, derived from balanced chemical equations and limiting reactants, forms the benchmark against which real-world results are measured. Missteps in either step can distort conclusions, undermining scientific rigor.
Defining Actual Yield: Measuring What You Actually Collect
Actual yield represents the mass or volume of product isolated from a chemical reaction—what chemists refer to as “the recovery.” This value is not immediately available; it requires careful experimental procedure. After a reaction completes, pure product is isolated—often via filtration, evaporation, or centrifugation—then weighed using a calibrated balance. For liquids, precise pipetting into a vessel is critical; even minor spillage or evaporation errors skew results.For solids, drying at controlled temperatures eliminates moisture, ensuring dry mass reflects true content. But measuring actual yield goes beyond mere weighing. Units must align with reactants and products—the balance must display power in grams, milligrams, or milliliters consistently.
For example, if a reaction produces 12.5 grams of sodium acetate trihydrate, but the balance reads in kilograms, a 10% error happens instantly. Analytical techniques like titration or spectroscopy may supplement balance data, especially for colored or unstable compounds—providing cross-verification that strengthens results.
Always report actual yield with clear, significant figures—never round prematurely.
If laboratory balances show three decimal places but the reaction produces only two meaningful digits, report to two, preserving integrity without overstating precision.
Calculating Theoretical Yield: Theory Meets Reaction Stoichiometry
Theoretical yield is the ideal maximum amount of product expected from a balanced chemical equation, based on stoichiometric ratios and limiting reactants. To compute it correctly, start by identifying the balanced equation—this ensures accurate mole conversions.For a reaction like 2 H₂ + O₂ → 2 H₂O, one mole of oxygen yields two moles of water. But which reactant limits production?
Limiting reactant analysis is pivotal.
It is the substance fully consumed first, capping product formation. Suppose 2 moles of hydrogen react with 1 mole of oxygen: hydrogen limits production since 2 moles of H₂ require only 1 mole O₂. From stoichiometry, 2 moles H₂ produce 2 moles H₂O; thus, theoretical yield = 2 moles H₂O.
Convert moles to grams using molar mass: 2 × 18.015 g/mol = 36.03 grams theoretical yield.
If unequal quantities are used, recalculate based on limiting reactant. For instance, 3 moles H₂ with 1 mole O₂ still yields 2 moles H₂O because oxygen limits output.
Summing deviations prevents major miscalculations—small errors compound when applied across multiple reactions, skewing research accuracy.
A Step-by-Step Guide to Accurately Calculating Percent Yield
To determine percent yield without confusion or error, follow a systematic approach:- Verify Reaction Balancing and Limiting Reactant: Write the balanced equation. Identify which reactant will run out first—this defines theoretical output.
- Weigh Actual Product: Use a moisture-free balance; dry products at constant weight. Record mass in correct units, noting decimal precision.
- Calculate Theoretical Yield: Convert moles of limiting reactant to product moles via stoichiometric ratios.
Multiply by molar mass to convert to grams.
- Compute Percent Yield: Divide actual yield by theoretical yield, then multiply by 100. Express as a percentage with up to three significant digits.
Always cross-check unit consistency: actual yield in grams matches theoretical in the same unit. A 98% yield of 4.8 grams material suggests only 2% loss—minimal but measurable. Automated lab balances with ±0.0001 g precision further reduce uncertainty.
Common Pitfalls and How to Avoid Them
Even experienced researchers stumble over subtle issues in percent yield calculations. Recognizing these traps early prevents systemic errors.Unit Mismatches Devastate Accuracy
Balancing reaction moles against mathematical yield unit inconsistencies causes dramatic错误.If a reaction yields 0.25 moles but results are reported in grams, conversion fails—equivalent mass depends on molar mass, misapplied units distort percent yield beyond recognition.
Ignoring Losses Underestimates Efficiency
Solvent residue, clinging product, evaporation, or incomplete filtration often reduce actual yield. Forgetting these losses inflates percent yield artificially—what looks like near-perfect efficiency may stem from overlooked procedure flaws.Over-Rounding Distorts Real Results
Reporting percent yield with excessive precision—say, 98.74% from a 45


Related Post

Unlock Your Destiny: How Lucky Astrology Placements Decoding Your Cosmic Gifts Reveals Hidden Talents and Destiny

Discovering Ymovieshd: Your Ultimate Streaming Destination for Unforgettable Movie Experiences

Toyota Stout 2024 Pre-Orders Now Open in Canada: The All-New Electric Midsize x Drives Preview

Navigating Identity and Leadership: Anies Baswedan’s Resilient Journey Through Jurong Elementary and Beyond

