ISO 13485: The Global Gold Standard for Medical Device Safety and Quality
ISO 13485: The Global Gold Standard for Medical Device Safety and Quality
When life hinges on device reliability, adherence to international standards becomes non-negotiable. Among these, the ISO 13485 standard stands as the definitive framework ensuring medical devices meet rigorous safety, performance, and quality benchmarks throughout their lifecycle. Developed by the International Organization for Standardization (ISO), ISO 13485—officially titled “Medical devices — Requirements for quality management systems”—provides a structured, risk-based approach tailored to the unique challenges of the medical device industry.
Unlike generic quality systems, ISO 13485 integrates regulatory expectations with practical implementation, making it indispensable for global market access.
At its core, ISO 13485 functions as a comprehensive quality management system (QMS) specifically calibrated for medical device manufacturers, regulators, and healthcare providers. It mandates systematic processes—from initial concept and design to manufacturing, testing, labeling, and post-market surveillance—ensuring every phase adheres to patient-centric safety standards.
“ISO 13485 bridges the gap between technical excellence and real-world application,” notes Dr. Elena Marquez, senior regulatory specialist at MedTech Innovations. “It’s not merely a checklist; it’s a mindset that prioritizes patient well-being above all.”
The standard’s structure is built on three foundational pillars: risk management, documentation rigor, and continuous improvement.
Under ISO 13485:2016—the current version—the concept of risk-based thinking is central. Article 6 explicitly requires organizations to identify, evaluate, and control risks throughout product development. For example, a pacemaker manufacturer must assess electrical safety, material biocompatibility, and long-term reliability under physiological conditions, then implement controls to mitigate hazards before market release.
Risk Management: The Heartbeat of ISO 13485
Risk management under ISO 13485 is not optional—it’s a regulatory binding. The standard aligns closely with ISO 14971, the globally recognized standard for medical device risk analysis and management. Manufacturers must establish a documented risk management system encompassing:- Detecting hazards through failure mode and effects analysis (FMEA) or fault tree analysis.
- Quantifying risks via probability and severity assessments.
- Implementing risk controls such as design limits, redundancy features, or clear user warnings.
- Monitoring post-market data to update risk profiles dynamically. This structured approach minimizes product-related harm and supports regulatory submissions.
- Design and development validation reports.
- Manufacturing process validation and quality control tests.
- Change control documentation tracking all modifications.
- Traceability matrices linking requirements to test results and production lots. Every medical device batch must be accompanied by comprehensive technical documentation, enabling regulatory bodies to verify compliance during audits.
- Gap analysis to assess current processes against ISO 13485 clauses.
- Process mapping and standardization of critical workflows.
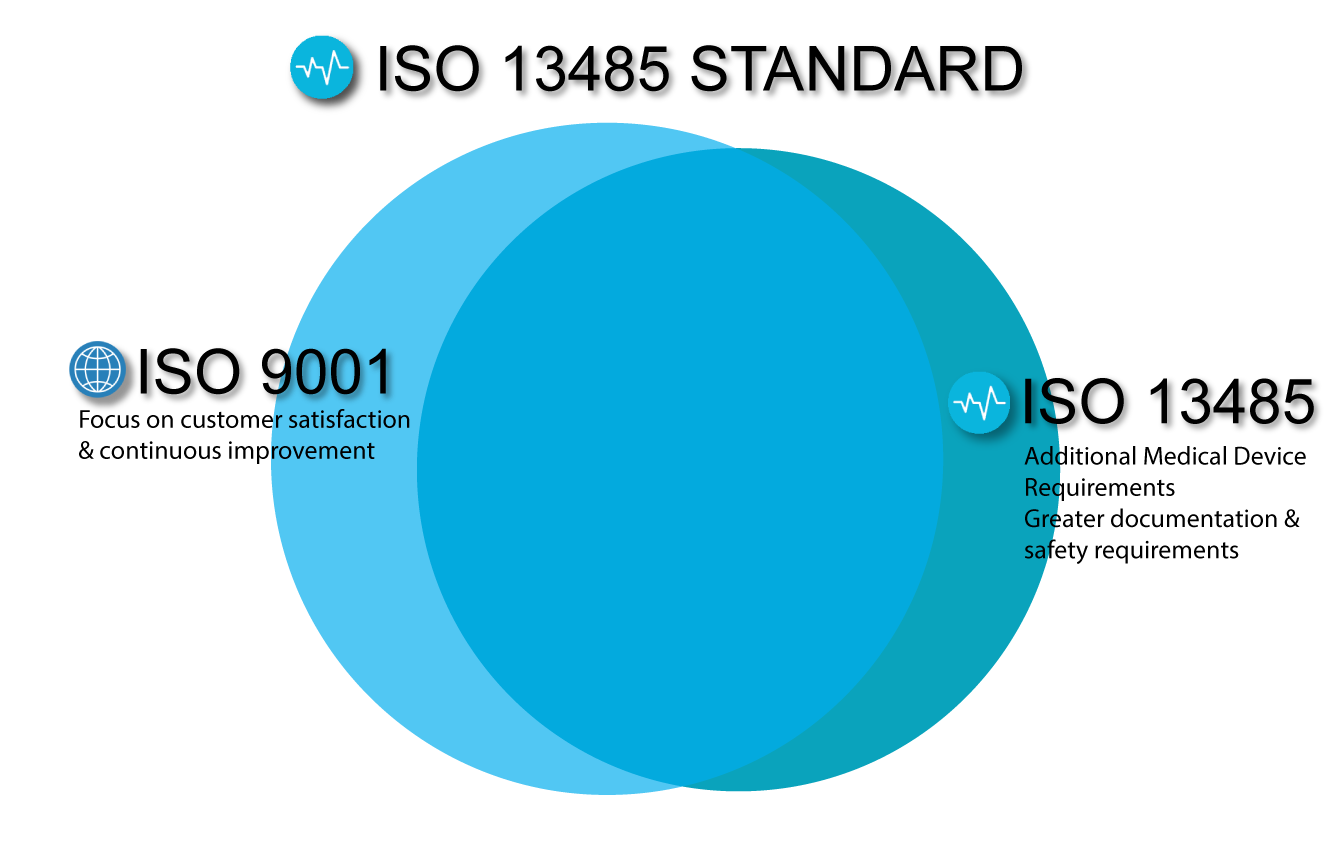
- Integration with existing quality systems (e.g., ISO 9001) to avoid silos.
- Internal audits and management review to confirm ongoing alignment.
- Certification by accredited bodies to validate compliance and build stakeholder confidence.
As Dr. Karl Anders, former ISO committee lead, states: “Effective risk management transforms uncertainties into measurable safety outcomes—critical for devices that interface directly with the human body.”
Documentation forms the backbone of ISO 13485 compliance, ensuring traceability, accountability, and transparency. The standard demands detailed records covering:
According to Dr. Marquez, “Without meticulous documentation, even the safest device loses credibility—auditors require proof, not promises.”
ISO 13485 also embeds the principle of continuous improvement, formalized through lifecycle monitoring and corrective action processes. Post-market surveillance is a critical expectation: companies must collect and analyze user feedback, adverse event reports, and field performance data.
When issues arise—as with certain orthopedic implants experiencing early degradation—manufacturers must initiate risk reassessments and implement timely corrective actions, often including recalls or design updates. This feedback loop not only protects patients but also enhances product evolution.
The standard’s influence extends beyond individual companies.
Regulatory agencies worldwide, including the U.S. FDA, EU Notified Bodies, and Japan’s PMDA, recognize ISO 13485 as equivalent to their own medical device QMS requirements. This harmonization facilitates global market entry, reducing duplication and accelerating access to life-saving technologies.
For manufacturers in emerging markets, adopting ISO 13485 isn’t just a compliance hurdle—it’s a strategic gateway to trusted international partnerships.
Implementing ISO 13485 demands organizational commitment, cross-functional collaboration, and ongoing training. The path typically involves:
The standard’s adaptability further solidifies its relevance in a rapidly evolving field. Recent updates address emerging challenges like digital health integration, software-as-a-medical-device (SaMD), and cybersecurity in connected devices. ISO 13485 now explicitly incorporates guidance on managing risks from artificial intelligence, IoT connectivity, and data privacy—ensuring it remains at the forefront of innovation.
Ultimately, ISO 13485 is more than a certification—it is a commitment to patient safety, technical excellence, and global responsibility. In an industry where failure is not an option, adhering to this standard isn’t just best practice; it’s the lifeblood of trust between makers and bearers of medical innovation. As industry leaders converge on this framework, ISO 13485 continues to set the gold standard for medical device quality—one rigorously certified product at a time.



Related Post

Ovo GitLab: Revolutionizing DevOps Collaboration with Seamless Integration and Unmatched Security

How Apple’s Rising Worth Net is Reshaping Investor Confidence and Market Perception

Unai Hernández at Barcelona: The Tactical Architect Reviving Catalan Football
Utah’s Current Time: Where Mountain Clocks Side With Mountain Time Standard

