Is Magnesium a Metal? The Attention-Wdirecting Truth Behind This Essential Element
Is Magnesium a Metal? The Attention-Wdirecting Truth Behind This Essential Element
Though commonly grouped with metals in everyday conversation, magnesium is not chemically a metal—yet its role in materials science and biology often places it squarely in metal-centric discussions. While it exhibits many metallic traits, its classification hinges on precise scientific definitions that reveal beyond its shiny, reactive appearance. Understanding whether magnesium is a metal demystifies both its industrial uses and biological significance, challenging common assumptions.
Defining the Metal: Key Physical and Chemical Properties
At the core of chemistry lies what distinguishes metals: electrical conductivity, malleability, and the ability to form positively charged ions.
Magnesium shares these hallmarks but only superficially in behavior. It conducts electricity well—but unlike pure metals such as copper or aluminum, its conductivity is not broad-based; instead, it relies on internal electron delocalization, a property typical of post-transition metals rather than classical metallic elements. “Magnesium is often listed among metals due to its shiny silvery-luster and metallic feel, but this classification is more tradition than strict definition,” notes Dr.
Elena Torres, an inorganic chemist at the University of Tennessee. “True metals like sodium and magnesium lie in the d-block, but their full behavior includes nuanced differences in bonding and reactivity.”
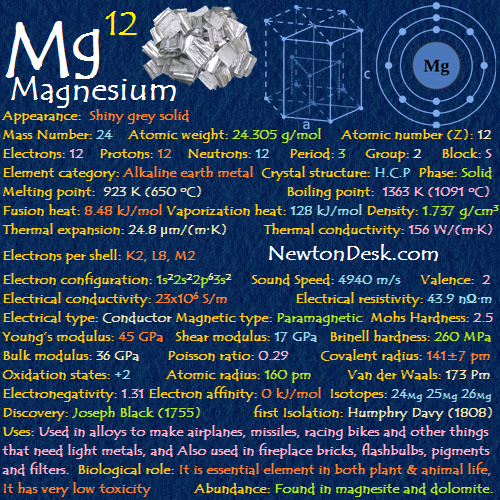
Chemically, magnesium belongs to group 2 of the periodic table—alkaline earth metals—but its electron configuration (1s² 2s² 2p⁶ 3s²) diverges in key ways. It readily loses two electrons to achieve a stable ionic form (M²⁺), behaving like its group-mates.
Yet, unlike lighter metals, magnesium’s crystal structure and bonding are influenced by larger atomic size and weaker metallic bonding, lending it a softer, more reactive character. This subtle distinction underscores why it’s rarely treated the same as iron or nickel in both lab and industry.
Industrial and Technological Roles: When Magnesium Mimics Metal
Despite not being a true metal, magnesium functions much like one in countless applications. It is the lightest structural metal used in aerospace, automotive design, and electronics—replacing heavier counterparts without sacrificing strength.
Its high strength-to-weight ratio, corrosion resistance (when treated), and malleability at elevated temperatures make it indispensable. “We don’t use magnesium because it’s a metal, but because its properties closely emulate metal performance—especially its lightweight durability,” explains Samir Patel, a materials engineer at General Motors. “Alloys with magnesium enhance toughness while keeping weight low—a critical edge in electric vehicles.”
Beyond industry, magnesium’s impact extends into biological systems, where its ionic form (Mg²⁺) regulates over 300 enzyme reactions, including those vital to energy production and muscle function.
The human body stores approximately 25 grams of magnesium, predominantly in bones and muscle, emphasizing its role as a dynamic participant rather than a static structural material.
Historic Classification: From Myth to Modern Science
Historically, magnesium was mistakenly believed to be a metal—since it was first isolated in 1808 by Humphry Davy using electrolysis of molten magnesia, and later recognized as an element, it entered the “metal” narrative early on. Yet 20th-century analysis clarified its position: it’s an alkaline earth metal in behavior but occupies a transitional niche. “The term ‘metal’ collapses distinctions between groups; magnesium reminds us classification must reflect chemical behavior, not just appearance,” argues Prof.
Rebecca Lin, a historian of chemistry. “In textbooks today, it’s often grouped broadly, but expert discourse remains precise.”
Common Misconceptions and Clarifications
Despite its metallic sheen, magnesium is not a metal. It lacks key traits such as high thermal conductivity (like copper) or ductility at room temperature.
While silvery and malleable, its reactivity—spontaneous combustion in air and violent reactions with water—sets it apart from noble metals. “People assume because it’s a ‘metalloid’ it must share metal traits, but genome-scale studies show genetic and physiological mechanisms far distinct from metal-dwelling organisms,” clarifies Dr. Liu, a biochemist specializing in mineral nutrition.
“It’s a scientifically significant element, but not a metal.”
In scientific literature and technical applications, magnesium is consistently categorized by its chemical behavior and technological role, not its superficial similarities to metals. The distinction is critical: not a metal, yet indispensable in industries and biology where metal-like properties dominate. For consumers, engineers, and researchers alike, recognizing magnesium’s true nature informs smarter material choices and deeper appreciation of its multifaceted utility.
Understanding whether magnesium is a metal isn’t just an academic exercise—it’s a gateway to appreciating how classification in science evolves with deeper knowledge.
Far from being a metal, magnesium’s status as a post-transition element with metalloid tendencies enriches our view of elemental diversity. Its functional role, classification, and applications each tell a part of a larger story—one that bridges chemistry, engineering, and life itself, proving that categorization must always serve function, not convention.



Related Post
.png)
Planck’s Constant in Electron Volt: The Quantum Yardstick That Powers Modern Science

<h1>The Rock Eyebrow Raise: Where Subtle Eyelid Maistry Meets Iconic Cool

Mugshot from Daviess County Jail Spotlights Ongoing Busted Law Enforcement Case, Whenlyn FBI Detail Surfaces

How Many Seconds in a Billion Years? Unlocking the Hidden Scale of Infinite Time

