How Many Protons Define Potassium? Unlocking the Atomic Identity Behind This Essential Element
How Many Protons Define Potassium? Unlocking the Atomic Identity Behind This Essential Element
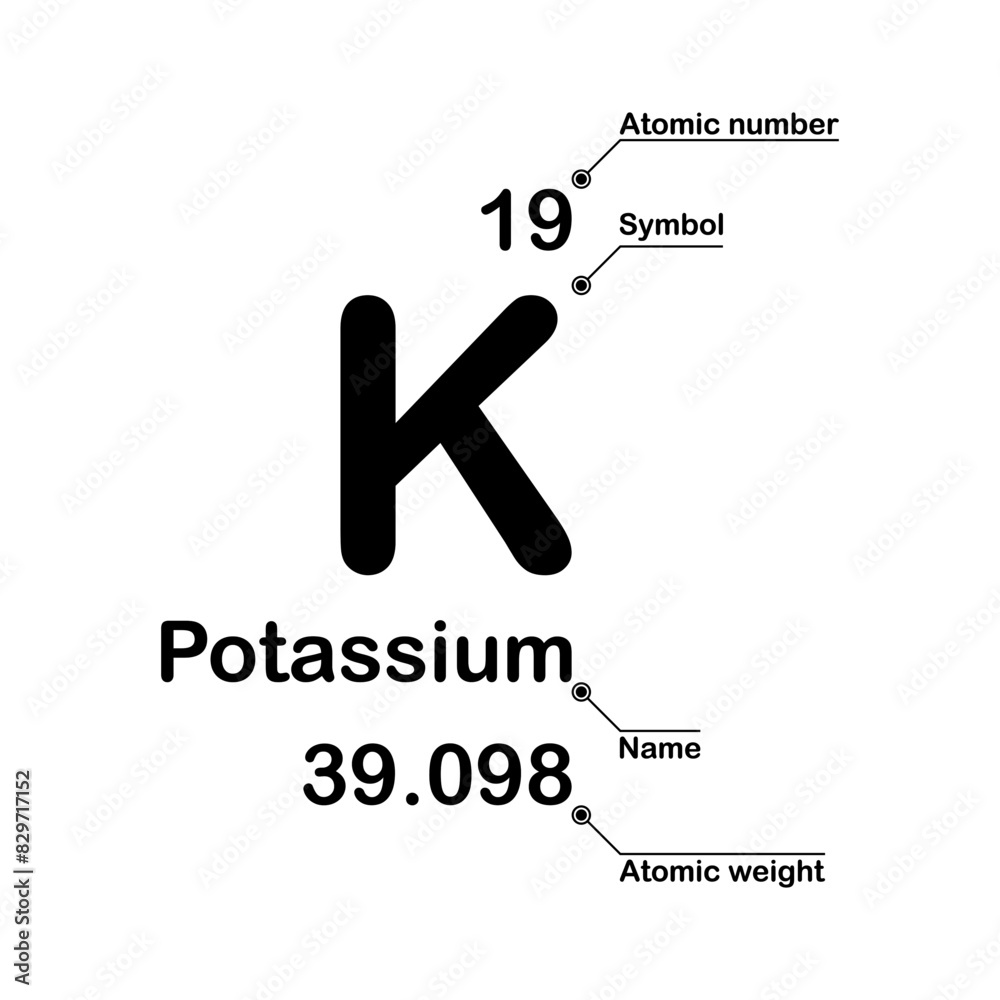
Potassium, a soft, silvery-white metal that tarnishes rapidly in air, plays a vital role in biology, industry, and chemistry—yet its atomic foundation remains a cornerstone of elemental understanding. At the heart of its identity lies a precise number of protons: 19. This single number determines potassium’s place in the periodic table, its chemical behavior, and its function in living organisms.
With exactly 19 protons in its nucleus, potassium belongs to the alkali metal group, a family renowned for reactivity and utility.
Each atom’s proton count defines its atomic number, a fundamental fingerprint that specifies the element. For potassium (atomic number 19), this means its nucleus contains 19 positively charged protons, counterbalanced by 19 electrons under atomic equilibrium.
This proton count directly governs potassium’s electron configuration and bonding character. Unlike neutral atoms, ions formed by gaining or losing electrons still retain the original number of protons—giving potassium a defining stability but enabling dynamic ionic interactions.
The Role of 19 Protons in Potassium’s Atomic Structure
The 19 protons within potassium’s core generate the strong positive charge that attracts electrons and orchestrates chemical interactions.Electrons orbit this nucleus in energy levels, and potassium’s signature electron arrangement—1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹—reflects its position in Group 1 of the periodic table. Here, the single valence electron in the 4s orbital is weakly bound, allowing potassium to readily donate it during reactions. This propensity for electron loss explains potassium’s famed reactivity, especially with water, driven fundamentally by its atomic structure centered on 19 protons.
“Potassium’s 19 protons create a distinct electronic landscape that fuels its chemical versatility,” notes Dr. Elena Ramirez, a senior inorganic chemist at the National Institute of Materials Science. “This single proton count shapes not only its atomic radius and electronegativity but also its role in biological systems and industrial applications.”
How Proton Count Influences Potassium’s Chemical Behavior
The proton number directly influences potassium’s chemical properties.With a relatively large atomic size and low ionization energy due to the single outer electron, potassium efficiently transfers electrons—forming stable K⁺ ions in aqueous or ionic environments. This behavior is critical in nerve impulse transmission, muscle contraction, and enzyme activation within living cells. Across industries, potassium compounds derived from its 19-proton core serve fertilizers, pharmaceuticals, and electronics, showcasing how atomic architecture scales to global utility.
Periodic Table Significance of Potassium’s Atomic Number
Sitting between calcium (20) and argon (18) on period 4, potassium’s atomic number 19 places it firmly in the alkali metals. This grouping is no accident—it reflects shared traits rooted in its core: 19 protons enabling the same valence electron dynamics across the series. “The proton count is nature’s blueprint,” explains chemist Dr.James Holloway. “Potassium’s identity is locked in its 19 protons, a detail that echoes throughout the periodic table and governs its entire chemical narrative.”
While potassium’s reactivity and applications capture public attention, the true essence lies in this proton foundation. It is the pivot around which electron arrangements, chemical reactivity, and periodic trends revolve—making the number 19 not just a statistic, but the core of potassium’s penetrating chemical identity.



Related Post

Another Word For Good: Transforming Challenges Into Meaningful Change

Meet the Unforgettable Icons of FNAF: Guardians, Monsters, and the Soul of Survival Horror

La Era del Hielo 5: Doblaje Chileno Lleva “Una Aventura Helada” a un Nuevo Nivel

Sabreena Brar: Redefining Social Media Influence in the Digital Age

