How Bohr Revolutionized Atomic Theory: Lighting the Path to Quantum Understanding
How Bohr Revolutionized Atomic Theory: Lighting the Path to Quantum Understanding
The story of the atom’s inner workings reached a defining turning point in 1913, when Niels Bohr introduced a model that redefined physics and cemented quantum theory in the scientific consciousness. At a time when classical mechanics faltered against the mysterious behavior of subatomic particles, Bohr dared to merge Rutherford’s nuclear atom with Planck’s quantum hypothesis, launching a theory that transformed how we perceive matter at its most fundamental level. His revolutionary shell model explained not only atomic stability but also the precise spectral lines emitted by elements—ushering in a new era of precision and insight into atomic structure.
Bohr’s atomic model emerged amid a growing crisis in 19th-century physics. Despite Rutherford’s identification of a dense nucleus in 1911, classical electrodynamics predicted that orbiting electrons would collapse into the nucleus, emitting energy inexorably and rendering atoms unstable. This stark contradiction demanded a radical new framework.
Niels Bohr, drawing on the nascent quantum ideas of Max Planck and Albert Einstein, proposed a bold solution: electrons occupy discrete, stable energy levels—quantized orbits—where they do not radiate energy, contradicting classical expectations but aligning with experimental observations. Central to Bohr’s breakthrough was the concept of energy quantization. He postulated that electron motion is restricted to fixed shells or levels, each corresponding to a unique energy state.
“Named levels,” Bohr explained, “are spatially defined regions around the nucleus where electrons may be found with certainty.” When an electron jumps between these levels, it absorbs or emits a precisely quantized amount of energy—exactly matching the observed spectral lines. This principle directly accounted for the hydrogen spectrum, particularly the famous Balmer series, providing empirical validation where prior theories had only failed. Another key innovation was Bohr’s incorporation of angular momentum quantization.
He ruled that orbital angular momentum must be an integer multiple of a fundamental quantum: ħ/2 (where ħ is the reduced Planck constant). “Only certain orbits are allowed,” Bohr asserted, “and these define the atom’s spectral character.” This quantized rule resolved inconsistencies in angular momentum behavior and laid groundwork for future quantum mechanical models, including Schrödinger’s wave equation. Bohr’s model, though limited—failing to explain multi-electron atoms or fine spectral structure—was a pivotal bridge between classical physics and the emerging quantum paradigm.
It introduced core concepts that remain essential: energy quantization, stable electron orbits, and photon emission/absorption during transitions. “Bohr didn’t just describe atoms,” noted physicist David Bohm, “he introduced a way of thinking about atomic behavior grounded in fundamental physical principles.” Historical analysis reveals that Bohr’s synthesis was not merely theoretical; it was experimentally driven. His use of the Rydberg formula and experimental spectroscopic data transformed abstract postulates into verifiable predictions.
This fusion of theory and experiment set a new standard for physics research. Moreover, Bohr’s insistence on complementarity—the idea that particles exhibit both wave-like and particle-like properties—deepened the conceptual foundation of quantum theory, influencing generations of scientists. The legacy of Bohr’s atomic theory extends far beyond early 20th-century physics.
It paved the way for semiconductor technology, laser physics, and quantum computing by providing a framework to manipulate electron transitions. Modern atomic clocks, atomic spectroscopy, and even materials science rely on principles first articulated in Bohr’s model. His work demonstrated that atomic structure is not random chaos but governed by elegant, quantized laws.
In essence, Bohr’s atomic theory marked a quantum leap—not just in science, but in human understanding. By imposing order on the apparent randomness of atomic behavior, he revealed a universe structured by discreteness, probability, and deep symmetry. His model remains a cornerstone in physics education and research, a testament to the power of imagination grounded in rigorous observation.
Bridging Classical Mechanics and Quantum Reality
Bohr’s model emerged at a critical juncture when classical physics faltered, unable to explain atomic-scale phenomena. The failure of Newtonian mechanics and Maxwell’s equations to describe electron dynamics prompted a reevaluation of physical laws. Rutherford’s nuclear model, while groundbreaking, lacked a stable framework for electrons; without quantization, orbiting electrons would collapse, an outcome contradicted by observed atomic stability.This inconsistency demanded a paradigm shift. Bohr addressed it by borrowing Planck’s quantum hypothesis—energy emission occurs in discrete packets rather than continuously. “This was radical,” recalled historian of science Clara Wechsel, “because energy radiating from an orbiting charge penalizes classical orbits, yet stability was obvious in experiments.” Bohr’s key innovation was therefore dual: quantized angular momentum (L = nℏ/2) and fixed energy levels corresponding to spectral lines.
Mathematically, Bohr derived electron orbits where angular momentum is quantized in integer multiples of ℏ, leading to force balance: centripetal force equals electrostatic attraction scaled by energy. Edward Condon later summarized: “Bohr’s insight was that electrons only occupy certain orbits—each labeled by a quantum number—thereby preventing collapse and enabling spectral precision.” No classical orbit satisfied both energy quantization and stability; only quantum levels could explain observations. The validation came swiftly.
Bohr accurately predicted hydrogen line wavelengths, confirmed by high-resolution spectroscopy. His model’s success transformed quantum ideas from speculative hypotheses into testable theoretical scenarios, accelerating quantum mechanics’ development by figures like Heisenberg and Schrödinger.
Legacy and Lasting Impact on Modern Science
Bohr’s atomic model remains a pillar of quantum physics, shaping how scientists visualize and manipulate atomic-scale phenomena.The concept of discrete energy states underpins technologies ranging from atomic clocks—used in GPS systems—to quantum dots in display technology. The principle of electron transitions between levels governs laser operation, enabling applications in medicine, telecommunications, and computing. In education, Bohr’s model persists as a foundational teaching tool, illustrating quantization before venturing into more advanced formalisms.
It introduces students to quantum rules through experimental diplomacy—linking theory to observable spectra. “Students grasp quantum ideas not as abstract math, but as real photon emissions, tangible evidence of quantized energy,” observes educator Elsie Chen. Moreover, Bohr’s philosophical contributions—to comprehensiveness, complementarity, and the limits of classical description—continue to stimulate debate in quantum foundations.
His work reminds science that progress often arises at conceptual frontiers, where bold postulations meet empirical harshness. In summation, Bohr’s atomic theory catalyzed the quantum revolution, transforming fragmented spectral data into a coherent framework of quantized stability. It exemplifies how theoretical innovation, grounded in observation, drives scientific evolution.
As modern research explores quantum coherence, materials engineering, and quantum information, Bohr’s insights endure—timeless guides through the invisible world of atoms.

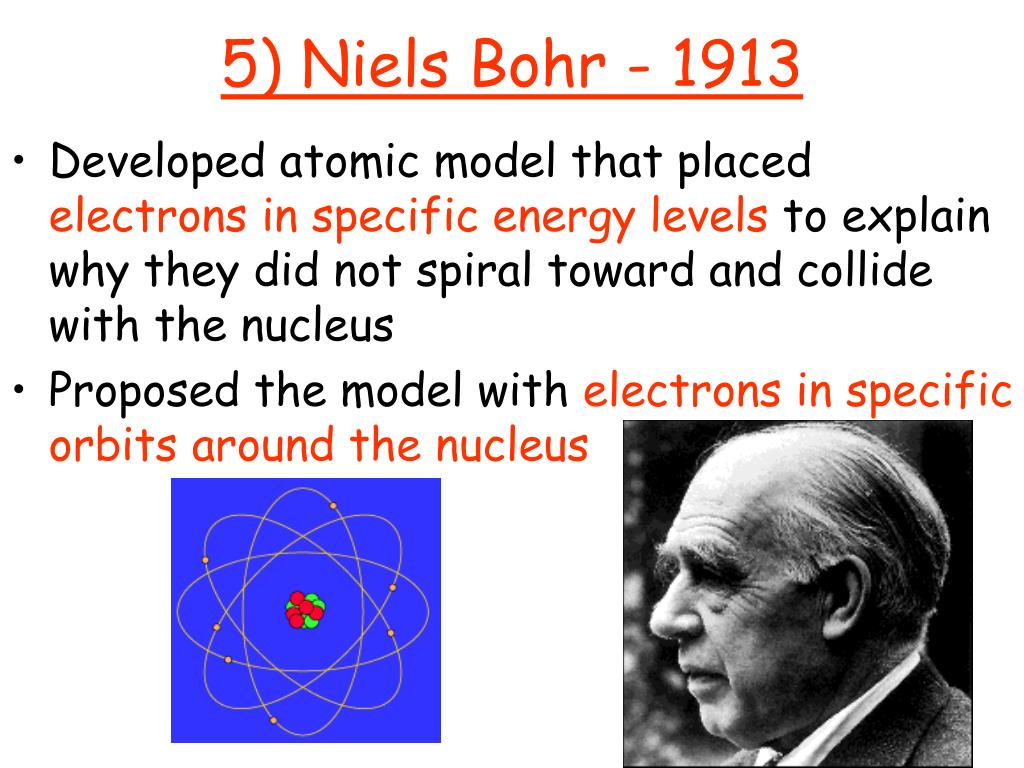
Related Post

Top AI News Sources You Must Follow to Stay Ahead in the Age of Intelligent Machines

Sayembara Artinya Artikel Jurnal Turasia Digelar Upaya Merawat Tradisi dengan Hasil Inovasi

William & Harry: A Royal Look Back Through Home Video Footage—Nostalgia in Every Frame

Unveiling Japanese Thin Double Eyelid Tape: The Ultimate Guide to a Precision-Crafted Eye Enhancement