Electrolysis and Electrolytic Cells: The Power Behind Chemical Transformation
Electrolysis and Electrolytic Cells: The Power Behind Chemical Transformation
At the heart of countless industrial and scientific processes lies electrolysis and the electrolytic cell—two fundamental technologies that unlock the transformation of chemical energy into usable forms. Through selective decomposition of compounds via electric current, these systems power everything from metal extraction and water splitting to industrial purification and energy storage. Understanding how electrolytic cells operate reveals not only the precision of modern electrochemistry but also its pivotal role in shaping a sustainable future.
The Core Mechanism: How Electrolysis Transforms Chemical Bonds
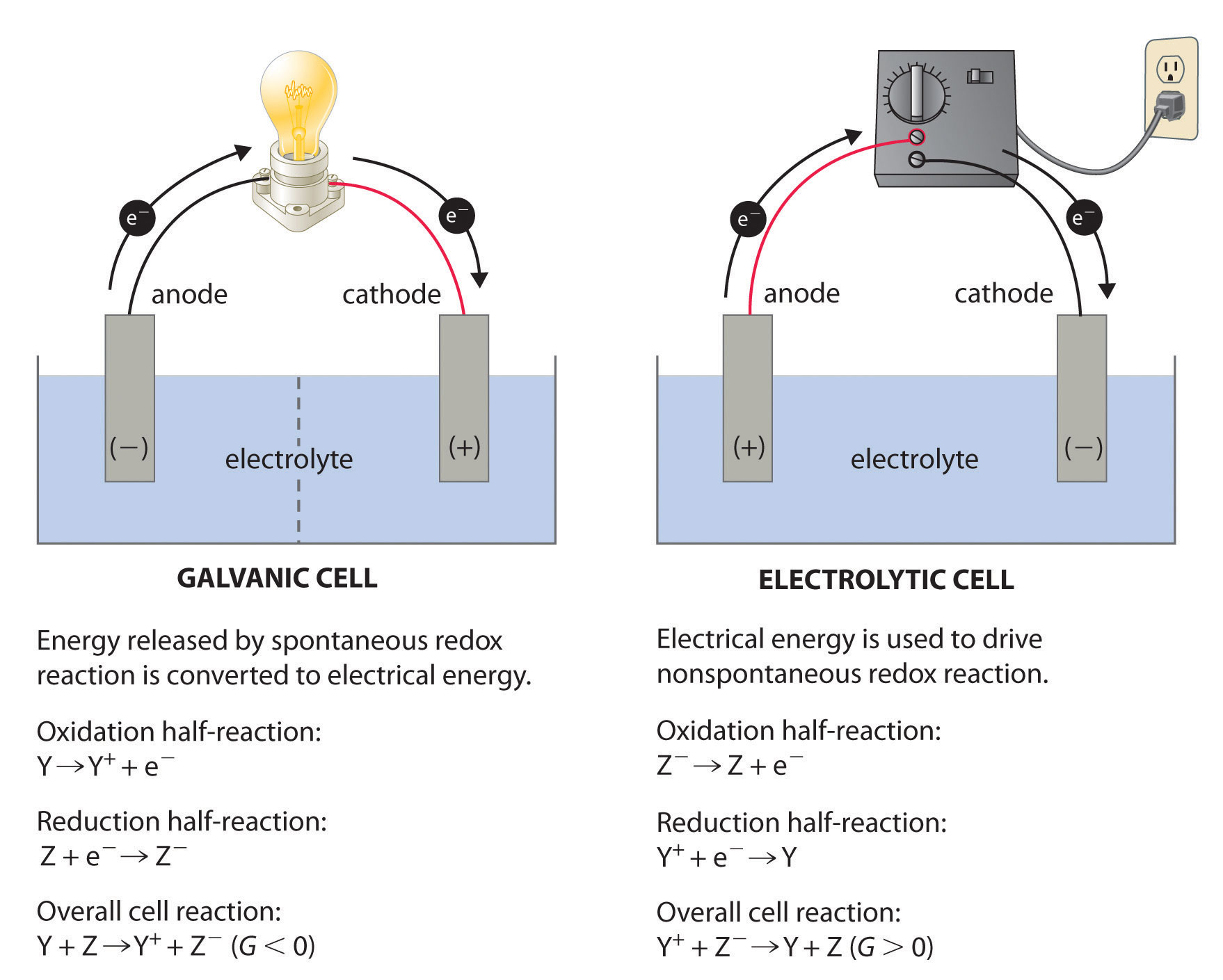
Electrolysis is the electrochemical process used to decompose ionic or polar compounds by driving a non-spontaneous redox reaction using an external electric current.
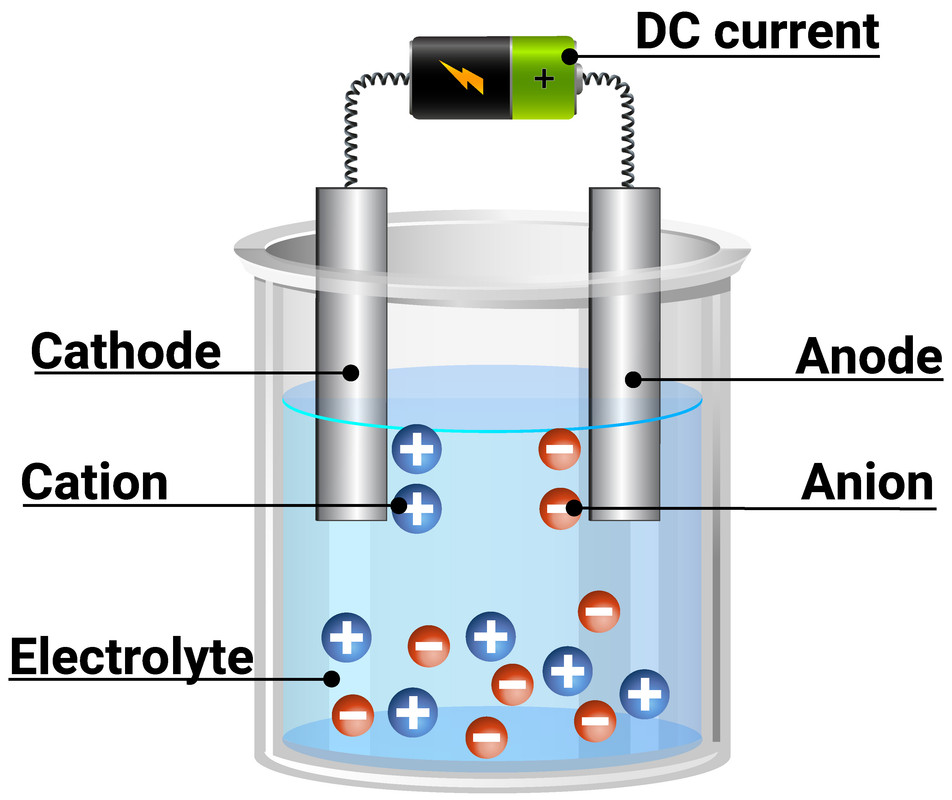
In an electrolytic cell, two electrodes—an anode and a cathode—immerse into an electrolyte solution saturated with charged species. When voltage is applied, oxidation occurs at the anode, releasing electrons into the system, while reduction takes place at the cathode, consuming electrons to drive chemical change. This controlled dissolution enables precise material transformations.
For instance, in aluminum production, aluminum oxide (Al₂O₃) dissolved in molten cryolite is reduced at the cathode to form molten aluminum, demonstrating electrolysis’s power to extract high-value metals from otherwise inert compounds. Similarly, water splitting into hydrogen and oxygen via electrolysis demonstrates its utility in clean energy: “Electrolytic water decomposition remains one of the most promising pathways to scalable green hydrogen,” notes Dr. Elena Marcus, electrochemical engineering expert at the Institute for Renewable Energy Systems.
“It decouples hydrogen production from fossil fuels, provided the electricity comes from renewable sources.”
The efficiency and selectivity of electrolytic processes depend on multiple factors: electrode material, electrolyte composition, applied voltage, and temperature. For example, in stainless steel pickling—an industrial process to remove surface iron oxide—chloride-based electrolytes accelerate oxide dissolution while minimizing unwanted metal poisoning. The redox reactions unfold as:
- At the anode: 2Cl⁻ → Cl₂(g) + 2e⁻ (oxidation)
- At the cathode: 2H₂O + 2e⁻ → H₂(g) + 2OH⁻ (reduction)
- Simple Single-Cell Setup: Used in small-scale lab experiments or battery electrolytes, where one electron transfer per ion drives measurable change.
- Industrial Stacks: Arrays of dozens to hundreds of cells connected in series or parallel to scale up reactions, as seen in large-scale aluminum or chlorine production plants.
- Membrane Cells: Incorporate ion-exchange membranes to separate products and control ion flow—essential in chlorine-alkali production, where sodium hydroxide, hydrogen, and chlorine are generated simultaneously.
This selective oxidation of halides protects the base metal, illustrating the precision possible with thoughtful cell design.
Designing the Electrolytic Cell: Practical Configurations and Applications
An electrolytic cell is more than just a vessel of conductive fluid; it is a carefully engineered system optimized for specific reactions.
Core components include two inert electrodes—often made of platinum, graphite, or stainless steel—configured to maximize surface area and minimize overpotential losses. The electrolyte, typically a molten salt, acid, or aqueous solution, must conduct ions efficiently while remaining chemically stable under operating voltages. Common configurations include:
Electrolytic cells power breakthroughs in resource recovery and clean energy.
Lithium-ion battery regeneration, for instance, relies on controlled electrolysis to recover lithium from spent cells. Similarly, in carbon capture efforts, electrolytic methods are being explored to reduce CO₂ to syngas or fuel molecules, turning waste into value. “Each electrolytic system is a precision instrument tailored to its mission,” explains Dr.
Marcus. “Whether isolating a pure metal or splitting water, success hinges on balancing chemistry, engineering, and energy input.”
Emerging applications push the boundaries further. Direct solar-to-fuel devices integrate solar panels with electrolytic cells to convert sunlight directly into hydrogen—an elegant solution to intermittency in renewable grids.
In mining, “in-situ leaching” uses electrolytic cells underground to extract minerals without excavation, reducing environmental disruption. These innovations showcase how foundational electrolytic technology adapts to address global challenges.
The Future of Electrolysis: Driving Sustainability Through Innovation
As the world pivots toward decarbonization, electrolysis and its cells emerge as linchpins of green chemistry and energy systems. The International Energy Agency highlights electrolytic hydrogen as a “cornerstone of long-term clean energy strategies,” particularly for hard-to-electrify sectors like heavy industry and long-haul transport.
Yet challenges remain: reducing energy intensity, extending cell lifetimes, and sourcing electricity sustainably are critical to maximizing environmental benefits. Researchers are responding with breakthroughs in materials science and process design. Novel catalysts—such as transition metal oxides and nanostructured surfaces—lower required voltages and improve reaction kinetics.
High-temperature electrolysis, using heat to boost efficiency beyond 90%, is gaining traction in industrial hubs. “Electrolytic cells are evolving from chemical workhorses into smart, adaptive systems,” states Dr. Marcus.
“Their role will only expand as electrolysis becomes smarter, cleaner, and more integrated.”
In sum, electrolysis and electrolytic cells are not mere lab curiosities—they are dynamic engines of chemical innovation. Powered by electricity and guided by precise electrochemical principles, they enable the extraction, purification, and transformation of matter with remarkable efficiency. As global demands for sustainable resource use and energy storage intensify, the evolution of these cells will continue to shape both industrial practice and environmental progress.
Their silent operation behind the scenes belies a profound impact—one that powers the future, atom by atom.


Related Post

Garfield Movies In Order: A Complete Filmography By Year — From Burgers to Blockbusters

Master BPJS Health Login for PKES Advantage: How E-Care EClaim Simplifies Excel E-Pcription & Vital Health Updates

Unveiling the Iconic Yellow Bronco Heritage Edition: A Legacy Rolling Through History

Discovering Lucy Liu Partner: Relationships & Private Life

