Diagram for Nitrogen Cycle: The Invisible Engine Sustaining Life on Earth
Diagram for Nitrogen Cycle: The Invisible Engine Sustaining Life on Earth
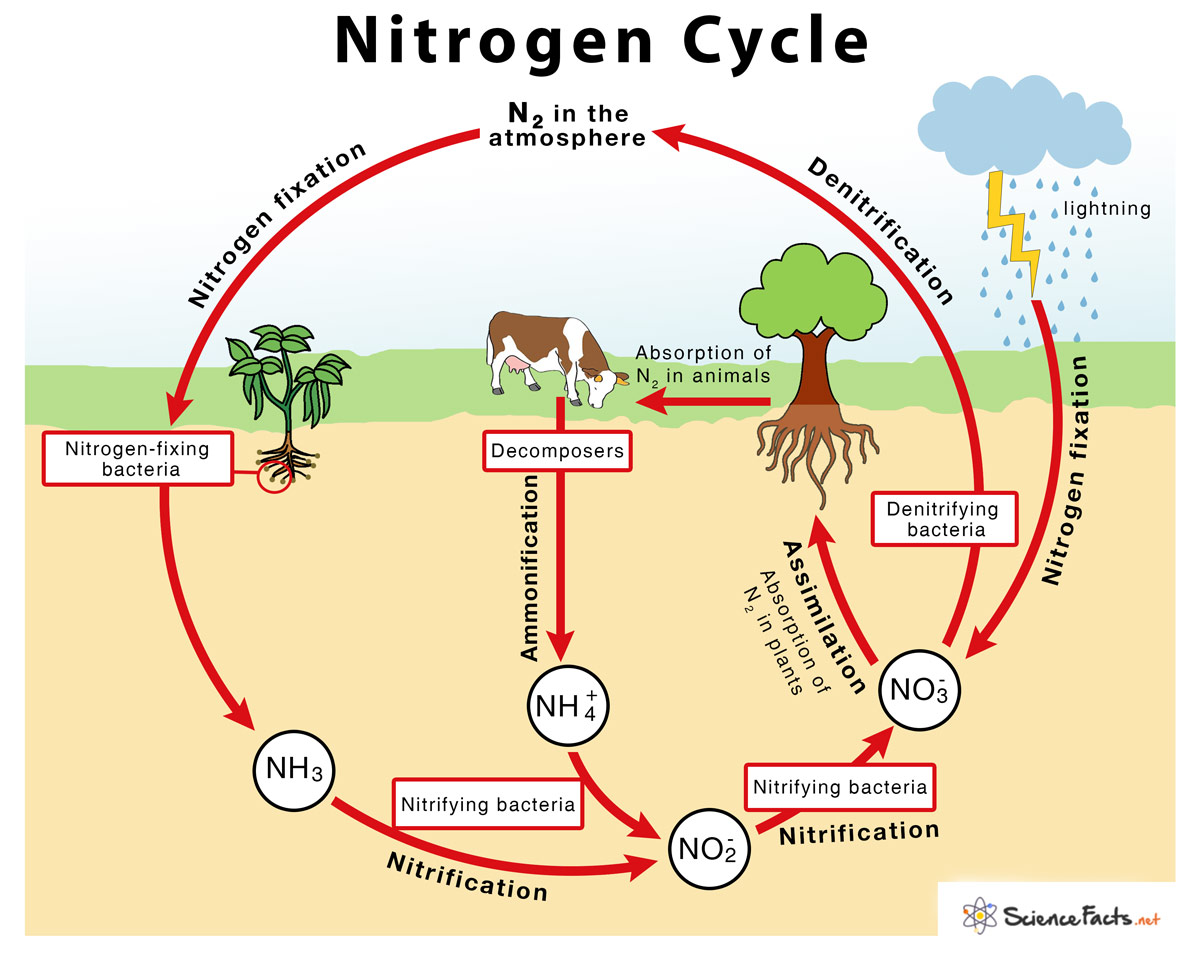
Nitrogen, the most abundant gas in Earth’s atmosphere, plays a paradoxical role: while vital for life, it exists predominantly in a form—molecular nitrogen (N₂)—that cannot be used directly by most organisms. Driving a complex, interdependent cycle, nitrogen cycles through the atmosphere, soil, water, and living beings via biological, chemical, and geological processes. This intricate web, illustrated visually in the diagram for nitrogen cycle, underpins ecosystem productivity and global biogeochemical balance.
Far more than a chemical pathway, the nitrogen cycle is a foundational pillar of biological sustainability—one that modern agriculture, climate regulation, and environmental health deeply depend on. The cycle begins with inert atmospheric nitrogen, which constitutes 78% of the air we breathe. But nature’s innovation lies in microorganisms capable of “fixing” this inert N₂ into biologically accessible forms.
Microbial Nitrogen Fixation: Nature’s Chemical Alchemy
Certain bacteria and archaea—such as symbiotic rhizobia in legume roots or free-living cyanobacteria in aquatic systems—convert atmospheric nitrogen into ammonia (NH₃) through enzymatic processes powered by metabolic energy. As bacterial microbiologist James Bohlen notes, “These microbes rewrite atmospheric chemistry, turning cosmic abundance into terrestrial life’s building block.” This transformation, nitrogen fixation, forms the critical entry point of nitrogen into ecosystems, enabling plants to synthesize amino acids, DNA, and chlorophyll. Once fixed, nitrogen undergoes a series of interlinked transformations: nitrification, assimilation, ammonification, and denitrification, each driven by microbial activity and environmental conditions.Four Key Stages in the Nitrogen Transformation Suite
- **Nitrification**: Aerobic bacteria like Nitrosomonas and Nitrobacter oxidize ammonia (NH₃) first into nitrite (NO₂⁻), then into nitrate (NO₃⁻)—a more mobile form readily absorbed by plant roots. - **Assimilation**: Plants uptake nitrate or ammonium ions through their roots, incorporating nitrogen into organic molecules essential for growth. Animals then consume plants, assimilating nitrogen into tissues and proteins.- **Ammonification**: Upon death and decomposition, organic nitrogen returns to the soil as ammonium (NH₄⁺) via the action of decomposers, recycling nitrogen back into the biotic loop. - **Denitrification**: Under low-oxygen conditions, specialized bacteria convert nitrate back into N₂ gas, completing the cycle by releasing nitrogen to the atmosphere—a process vital for balance but capable of contributing to greenhouse gas emissions when enhanced by human activity. Geochemical processes also shape the cycle on longer timescales.
Volcanic emissions release small amounts of reactive nitrogen compounds, while lightning strikes fix nitrogen through high-energy atmospheric reactions, producing nitric oxide (NO). These inputs, though minor compared to biological fixation, underscore nitrogen’s dynamic interplay with Earth’s geology and atmosphere. A visual diagram of the nitrogen cycle reveals the interconnectivity of these processes, mapping flows between reservoirs—like atmospheric N₂, soil nitrogen pools, and organic biomass—through microbial and physical pathways.
Mapping the Journey: Key Processes at a Glance
- Atmospheric N₂ ⇄ Soil: The central exchange, mediated by wind-driven deposition, microbial fixation, and leaching. - Soil Organic Matter: A reservoir where nitrogen is stored temporarily in decaying biomass. - Aquatic Systems: Rivers, lakes, and oceans facilitate nitrogen transport, transformation, and loss via denitrification zones.- Human Influence: Industrial Haber-Bosch process industrializes nitrogen fixation, doubling global reactive nitrogen inputs since 1900—altering natural balances. Environmental stability hinges on maintaining this equilibrium. Excess nitrogen from synthetic fertilizers and fossil fuel combustion fuels eutrophication in waterways, disrupts soil microbiomes, and accelerates nitrous oxide (N₂O)—a potent greenhouse gas with 300 times the warming potential of CO₂.
As ecologist David Tilman warns, “We have become planetary-scale geochemical engineers, reshaping a cycle that evolved over billions of years.” Understanding the nitrogen cycle through its diagram is not merely academic—it is essential for sustainable agriculture, clean water management, and climate mitigation. The nitrogen cycle exemplifies nature’s circular economy, where waste from one organism fuels another, sustaining ecosystems from rainforests to oceans. Its visualization guides scientists, policymakers, and stewards alike toward informed choices that preserve this irreplaceable planetary system.
In essence, the diagram for nitrogen cycle is far more than a biochemical schematic; it is a testament to life’s delicate balance, a map of invisible yet indispensable forces that define Earth’s habitability. Recognizing and respecting this cycle enables humanity to act as responsible custodians—not conquerors—of the planet’s life-support systems.



Related Post

Best Game Websites Shaping the Future of Digital Play: Where Innovation Meets Immersion

The Essential Edgenuity Pre-Calculus Blueprint: Unlocking Success in Pre-Cal
Anniston Doppler Radar: Watching Sky Swirl in Real Time Across Alabama

Ravalli County’s DUI Arrests Task Force Reveals Powerful Drop in Fatal Drunk Driving Calls

