Decoding H₂ with Lewis Dot Structures: The Molecular Blueprint of Hydrogen
Decoding H₂ with Lewis Dot Structures: The Molecular Blueprint of Hydrogen
In the foundational world of chemistry, understanding molecular geometry and bonding patterns is essential—and few molecules illustrate simplicity and precision like H₂, the diatomic hydrogen atom. Though seemingly elementary, the Lewis dot structure for H₂ reveals a precise and elegant picture of covalent bonding, electron sharing, and chemical neutrality. By mastering this core molecular model, scientists and students alike uncover the principles that govern reactivity, stability, and multiplicity in hydrogen’s behavior—key foundations in both life sciences and industrial applications.
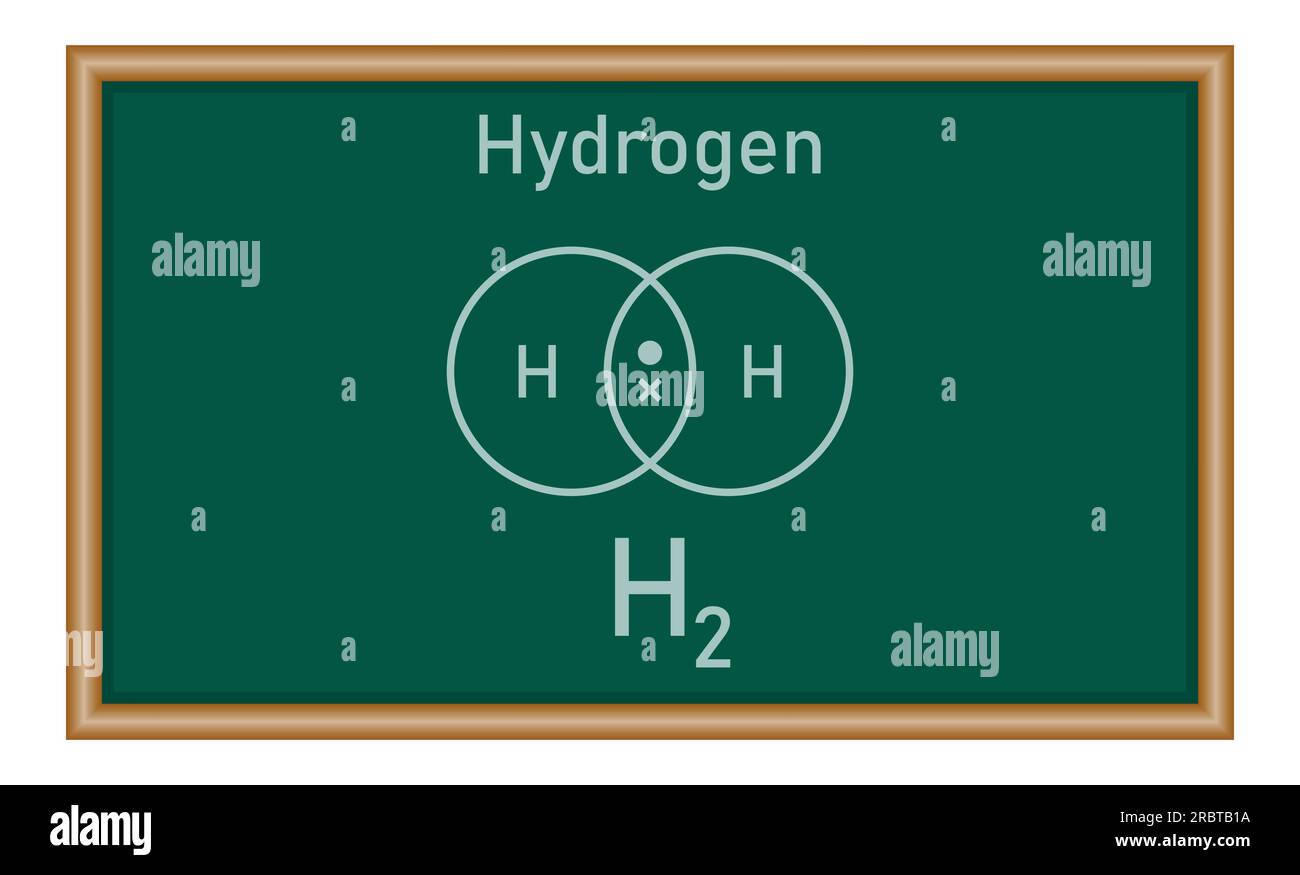
Lewis dot structures, named after chemist Gilbert N. Lewis, provide a clear visual representation of valence electrons and bonding arrangements. For the H₂ molecule, the structure centers on two hydrogen atoms, each contributing one valence electron from its single 1s orbital.
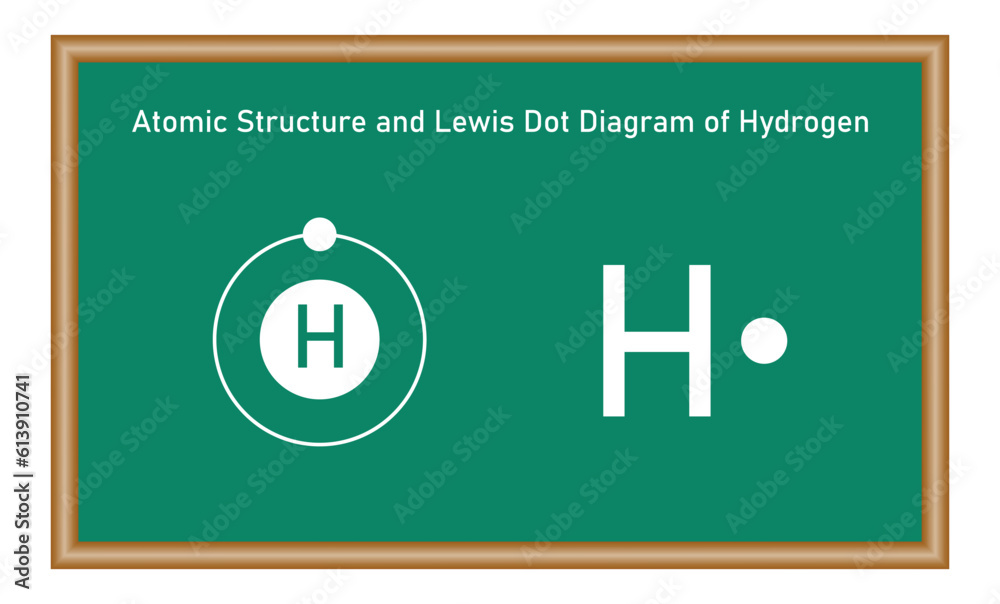
The simplicity of hydrogen—its atomic number of 1 and valence electron count of just one—demands a bonding pattern that prioritizes stability through electron sharing. This electron economy is elegantly captured in its dot configuration.
Building the Atomic Foundation of H₂
At the heart of H₂’s Lewis structure lies two individual hydrogen atoms, each displaying a single unpaired electron in its 1s orbital.These atoms are electrically neutral individually, with a total electron count of two—one per atom. To achieve the stable electron configuration of helium (He)—a noble gas with a complete outer shell—each hydrogen donates its only valence electron, forming a shared pair. This transfer, though ethereal in representation, symbolizes real electron movement through covalent bonding.
The resulting structure shows two dots clustered between the two yellow spheres, each dot representing one electron. These shared electrons form a single covalent bond, visually signaling how hydrogen achieves a duet electron configuration, satisfying the octet rule in a minimal, efficient format. Unlike more complex molecules with expanded octets or formal charges, H₂ maintains a serene simplicity, with no formal charges and full electron sharing.
By convention, the Lewis dot diagram for H₂ is rendered with: - → HT → (two dots between the atoms) - Individual hydrogen spheres labeled with electron symbols - No lone pairs, reflecting the molecule’s neutrality - A clean, balanced appearance emphasizing symmetry and stability
Electron Distribution and Geometric Implications
Though hydrogen is a small atom, its bonding moment reveals broader principles in molecular geometry. The single covalent bond in H₂ defines a linear molecular geometry—an angle of 180 degrees—where atoms align directly opposite each other along an axis. This geometry, though straightforward, underpins critical physical properties: H₂ is a nonpolar diatomic gas at room temperature, liquefiable under pressure, and inert under standard conditions due to its strong yet modest bond energy (~436 kJ/mol).“A formally neutral molecule with a single shared electron pair defines H₂’s peaceful bonding,” explains Dr. Elena Torres, a physical chemist specializing in molecular bonding. “There’s no angular distortion or lone-pair repulsion to complicate its structure—only pure electron sharing.” This simplicity starkly contrasts with polyatomic species that demand VSEPR theory or hybrid orbital models, underscoring H₂’s role as a baseline system in chemical education.
The absence of formal formal charges (∆±0) reinforces H₂’s stability. In Lewis notation, any deviation from a neutral 0 charge signals reactivity or instability—qualities H₂ lacks. The uniform distribution of electron density between the nuclei ensures equal sharing, eliminating bond polarity.
This symmetry not only defines its physical identity but also its behavior in chemical reactions, where H₂ acts consistently as a reducing agent or fuel source without complex charge-driven interactions.
Beyond Representation: Applications and Molecular Behavior
The utility of the H₂ Lewis structure extends far beyond static visualization. It serves as a template for understanding molecular orbital theory, reaction mechanisms, and catalytic interactions. For example, in ammonia synthesis (Haber process), knowledge of H₂’s electron behavior informs how nitrogen and hydrogen coordinate on metal catalysts.The molecule’s unreactive yet reactive duality—each hydrogen ready to bond, yet stable in the diatomic form—makes it ideal for energy applications like hydrogen fuel cells. Moreover, the Lewis dot schematic enables rapid assessment of stability in radical reactions. While free hydrogen atoms (H•) are highly reactive due to unpaired electrons, H₂’s paired electrons render it exceptionally stable.
This dichotomy highlights the significance of electron pairing in molecular resilience. As chemistry professor James Lin notes, “H₂ stands as a textbook case: simplicity breeds robustness. Its structure is not just a drawing—it’s a predictive tool.”
In industrial processes, such clarity streamlines design.
Whether scaling up hydrogen liquefaction, fuel delivery systems, or synthetic pathways, engineers and chemists rely on this foundational model to anticipate bonding strength, phase behavior, and interaction energies. The clarity of its Lewis structure allows scientists to bypass complex quantum calculations in preliminary stages, accelerating innovation without sacrificing accuracy.
Comparative Insight: H₂ Among Molecular Systems
To appreciate the uniqueness of H₂’s Lewis structure, contrast it briefly with other diatomic molecules. O₂, for instance, features two unpaired electrons in antibonding orbitals, resulting in paramagnetism and weaker bond order.F₂ contains filled bonding and antibonding subbands, affecting its polarity and reactivity. In contrast, H₂’s absence of antibonding electrons and zero formal charge yields maximal stability and inertness—until disrupted by external energy. The minimal electron count and lack of lone pairs make H₂’s structure a rare example of inherent covalent harmony untainted by strain or electron excess.
| Property | H₂ | O₂ | F₂ | |----------------|----------------|------------------|------------------| | Valence electrons| 2 total (1 each)| 12 total (6 each)| 18 total (7 each)| | Bond order | 1 | 2 | 1 | | Formal charge | 0 | 0 | 0 | | Polarity | Nonpolar | Paramagnetic | Polar | | Reactivity | Low, stable | Moderate | High, reactive | | Electron configuration | (1s²) shared | (2p⁴ 2p⁴) split | (2p⁵ 2p⁵) filled | This table underscores how H₂’s electronic architecture—clean, balanced, and fully paired—defines its niche in chemistry’s vast landscape. It is, in essence, the purest example of covalent harmony.
In the broader tapestry of chemical bonding, few molecules exemplify precision, stability, and elegance as clearly as H₂.
The Lewis dot structure crystallizes a fundamental truth: effective chemistry often starts with simplicity. By revealing just two electrons shared between two atoms, it teaches the deeper principles of electron economy, neutrality, and bond strength. As science advances, this humble structure remains indispensable—not only in classrooms but across laboratories and industries shaping the future of energy, materials, and molecular design.
The story of H₂ is not just about hydrogen; it is about how minimalism fosters mastery in chemistry.

Related Post

Jeep Catalytic Converter Issues: A Comprehensive Guide to Diagnosis, Causes, and Solutions

Bobby Flay’s Wife: Behind the Celebrity Chef’s Private Love Life

Breaking Bad Jane: The Quiet Power Behind the Longest Season of One

The Cast That Defines The Lincoln Lawyer: Unpacking the Icons Behind the Legal Drama

