Decoding Fluorine’s Lewis Structures: How a Simple Atom Reveals Deep Chemical Insights
Decoding Fluorine’s Lewis Structures: How a Simple Atom Reveals Deep Chemical Insights
At the heart of modern chemistry lies a surprisingly powerful tool: the Lewis diagram. Nowhere is this more evident than in the case of fluorine — the most electronegative element, whose unique bonding behavior defies conventional expectations. The Fluorine Lewis Diagram is not merely a sketch; it is a revealing map of valence electrons, molecular connectivity, and reactivity patterns that shape the chemistry of life and materials alike.
By analyzing fluorine’s electron configuration and bonding logic, scientists decode how this tiny atom orchestrates complex chemical interactions, influencing everything from pharmaceutical design to industrial catalysts.
The Fluorine Lewis Diagram: A Window into Electron Behavior
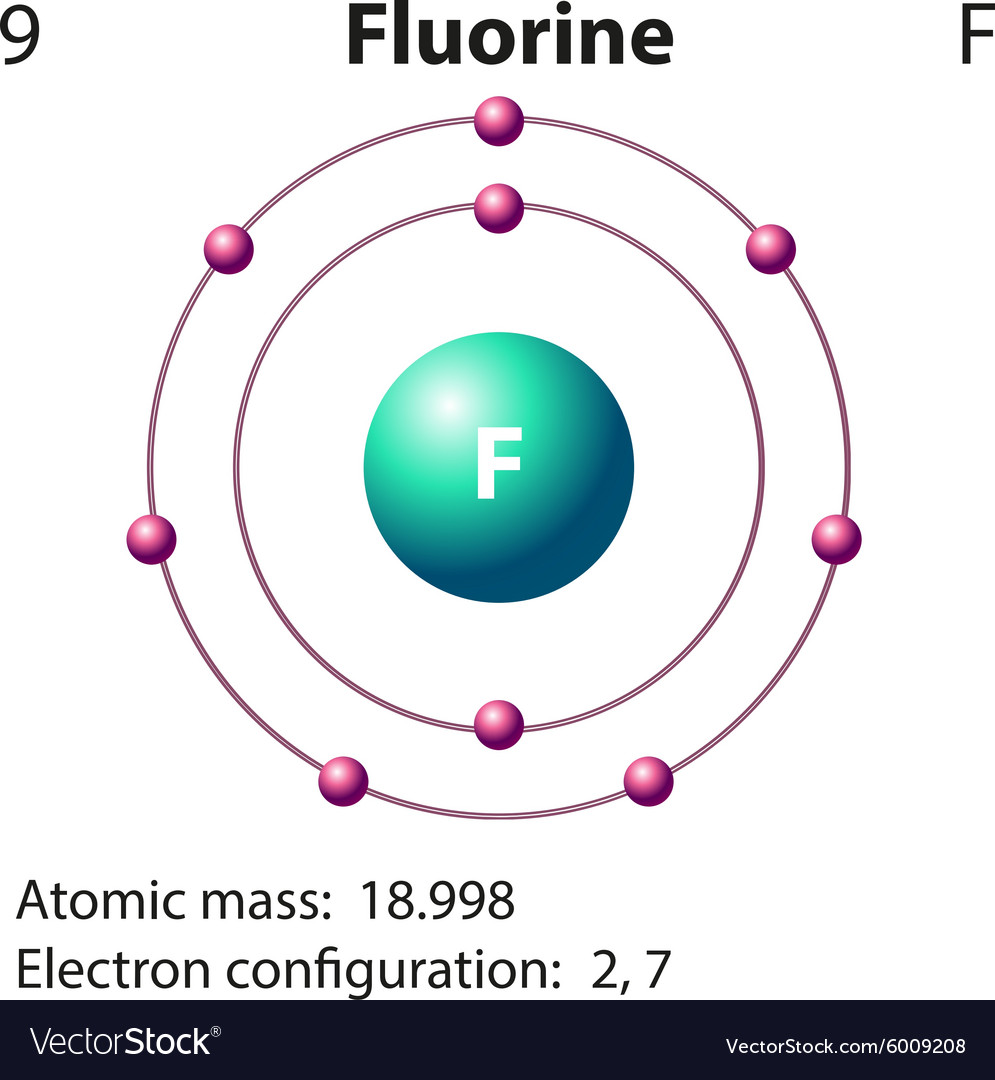
Fluorine, with atomic number 9 and electron configuration 1s² 2s² 2p⁵, occupies a special place in the periodic table. Its valence shell holds seven electrons, making it only one electron short of achieving a stable noble gas configuration.This inherent electron deficiency drives fluorine’s distinctive chemistry. The Lewis structure for fluorine—though it typically appears as a monatomic atom—offers a foundational insight into its bonding strategies. Since fluorine rarely forms molecules as a lone atom (it tends to exist diatomically, F₂), its Lewis diagrams focus on interactions with other elements.
In every compound, fluorine’s electrons are redistributed to achieve full octets, emphasizing its strong electronegativity and reactivity.
| Atom | Fluorine (F) | Electron count (valence shell) | 7 electrons | Electronegativity (Pauling scale) | 4.0 |
|---|---|---|---|---|---|
| Bonding | Uses shared or transferred electrons to complete octet | max 8 valence electrons in bonded species | forms single, double, or higher-order bonds in compounds | ||
| Key Feature | The empty p-orbital availability allows fluorine to accept one electron and stabilize in compounds like hydrofluoric acid (HF) or organofluorine pharmaceuticals | ||||
| Fluorine atoms in drug molecules enhance metabolic stability, lipophilicity, and binding affinity. Common drugs like Prozac (fluoxetine) and Keytruda (pembrolizumab) rely on fluorinated moieties to achieve targeted action. | Fluorine’s Lewis-driven electron density affects pKa and pI, critical parameters in drug absorption and distribution. | Examples include antivir
Related Post
Is Aliexpress Truly Safe and Reliable in 2024? The Full Breakdown on Reviews and Risks
Lions vs 49ers: Key Player Stats That Decided the Default Faceoff in Top-Field Clash
Who’s Unraveling Newswatch 16? The Whirlwind Departure at Wnep Newswatch 16
Master the Interview: Ace Tech Questions That Separate Top Candidates
Elsa and Anna: Unbreakable Sisters, Magical Bonds That Transcend Ice and Emotion |
