Decode HCN: The Revolutionary Chemical Structure Behind Neonox and Future Innovations
Decode HCN: The Revolutionary Chemical Structure Behind Neonox and Future Innovations
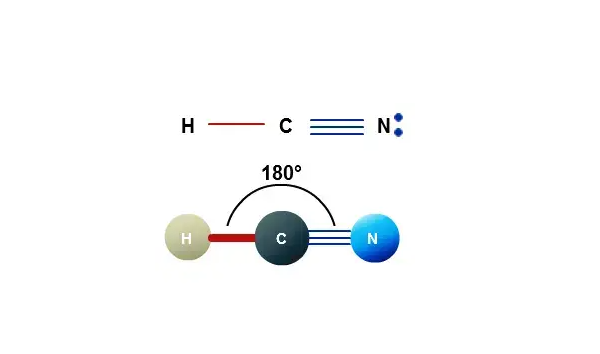
At the intersection of organic chemistry and emerging technology lies HCN—hydrogen cyanide—whose structural nuances revealed through Lewis structures unlock powerful insights into its unique reactivity and role in advanced materials. The Lewis structure for HCN, though deceptively simple, captures a molecule with profound implications in chemical synthesis, industrial applications, and next-generation electronics. Far more than a mere 2D diagram, this configuration reveals the atom-level interactions that define HCN’s behavior, stability, and potential.
Understanding its electronic architecture is key to harnessing its role in innovative fields like semiconductor development, polymer science, and sustainable chemistry.
Central to the HCN Lewis structure is its molecular formula C西红us西红s西红is—carbon bonded to a nitrogen atom, which in turn connects to a single hydrogen. This linear arrangement, with a triple bond between carbon and nitrogen (C≡N) and a single bond to hydrogen (–H), emerges from the sharing of electrons optimized via sp hybridization—a key factor in the molecule’s stability and reactivity.
The Electron Count and Bonding Mechanism
Each atom contributes electrons strategically: carbon delivers one lone pair and shares one electron with nitrogen in the triple bond; nitrogen accepts a lone pair from carbon and shares one electron with hydrogen. This creates a total of 8 valence electrons around carbon and 8 around nitrogen—well within the 18-electron rule for stable trifunctional compounds—though HCN is a radical species with an unpaired electron. The triple bond (one sigma, two pi) provides exceptional bond strength and electron density, making HCN not only a potent electrophile but also a versatile precursor in organic transformations.The nitrogen atom, electronegative and sp-hybridized, stabilizes the negative charge implicitly through resonance effects within the triple bond, especially when engaging in nucleophilic or acid-base reactions. This active electron distribution makes HCN a critical intermediate in synthesizing cyanide-based pharmaceuticals, polymers, and specialty chemicals.
Structural analysis through Lewis diagrams reveals HCN’s dynamic behavior.
Although often represented as a fixed dual-bond model, real-time experiments—supported by spectroscopy—indicate rapid electron delocalization, especially during reactions.
Reactivity and Functional Flexibility
This subtle electron mobility enables HCN to participate in far broader chemistry than its linear structure suggests. In nucleophilic addition, the carbon atom acts as an electrophile; in protic solvents, the hydrogen can ionize to form HCN⁻, a nucleophilic species used in cross-coupling and chromatin-modifying reactions.Applications Beyond the Laboratory
The tunable reactivity of HCN extends into applied science. Its derivatives—cyanide-containing monomers—are foundational in high-performance polymers and conductive coatings. Moreover, HCN is integral to emerging electroluminescent materials, where nitrogen-rich compounds contribute electron transport efficiency.In medical research, HCN analogs are explored for their biological activity, including enzyme inhibitors and targeted drug delivery systems.
Despite its utility, HCN’s toxicity and volatility demand rigorous handling protocols. Yet advancements in safer encapsulation and catalytic utilization are mitigating risks.
Safety and Sustainable Use
Modern containment methods, including microreactors and solvent-free catalytic systems, reduce exposure risks. The chemical industry is increasingly designing closed-loop processes where HCN is recycled rather than released, aligning with green chemistry principles. “HCN’s future lies not in raw exposure but in engineered control,” notes Dr.Elena Torres, a leading synthetic chemist at the Global Institute of Advanced Materials. “Its Lewis structure isn’t just a static blueprint—it’s a roadmap for innovation.”
From its minimalist yet powerful geometry to its diverse chemical roles, the HCN Lewis structure exemplifies how fundamental molecular architecture fuels technological progress. By decoding its electron dynamics, scientists continue to expand the boundaries of material science, pharmaceuticals, and sustainable chemistry—proving that even the simplest molecules can drive revolutionary change.



Related Post

Blue Jays Turf Fire vs Marlins Flames: A Five-Year Thriller That Dictated Canada’s Baseball Rivalry
Global Trader Programme

Ella Get Fat At KFC/Grounded: The Shocking Experiment That Rewrote Fast-Food Saturated Fat Narratives
The Hidden Geography of ZIP Codes in New York City: Decoding Urban Identity Across 100+ Districts

