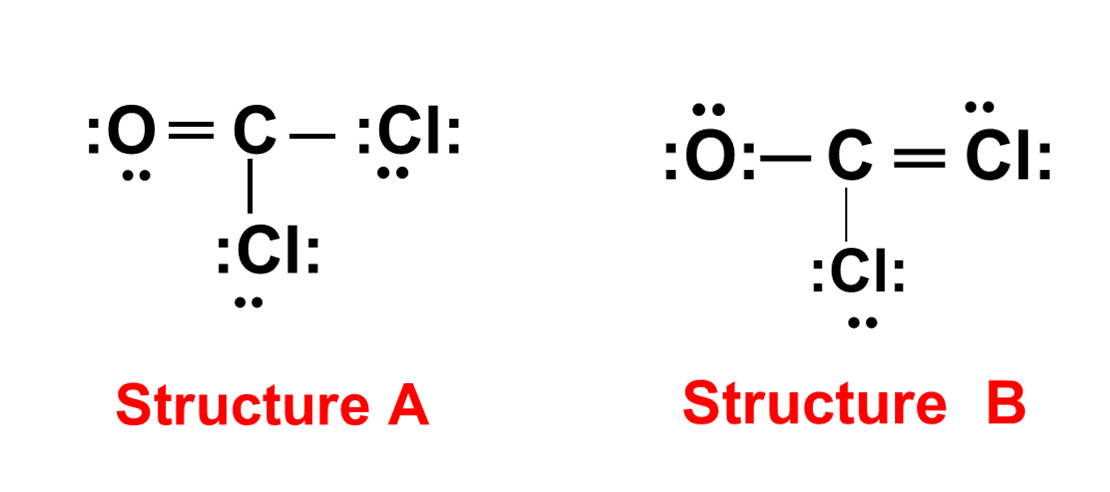
Cocl2 Lewis Structure
Unlocking Insight: The Chemistry Behind Cocl₂ and Its Lewis Structure Reveals Critical Insights into Molecular Behavior
The molecular architecture of Cocl₂, defined by its precise Lewis structure, lies at the heart of understanding a compound with growing importance in both industrial and medicinal chemistry. Though often overshadowed by more famous inorganic systems, the structural arrangement of Cocl₂ offers profound implications for reactivity, stability, and application potential. By analyzing its electron distribution and bonding pattern, researchers uncover fundamental principles that guide synthetic design and functional optimization in advanced materials and pharmaceuticals. Cocl₂, a dimeric halogen compound, features a central structural framework in which two Cocl units are linked through covalent bonds governed by the principles of electron pair sharing.At the core of its chemical behavior is its Lewis structure — a diagram that maps connecting atoms and localized electron pairs, revealing both bonding and non-bonding interactions. The precise geometry, determined by valence electron count and repulsion minimization, reflects a tetrahedral or planar arrangement depending on hybridization and lone pair effects.
Core Electron Architecture: Implications of Cocl₂’s Lewis Structure
Cocl₂’s Lewis structure demonstrates two central halogen atoms connected by a covalent bond, each surrounded by four regions of electron density.This corresponds to sp³ hybridization in the central atoms, yielding a tetrahedral electron geometry. However, actual molecular shape deviates slightly due to lone pair-bond pair repulsions, leading to a distorted tetrahedral or, in some cases, trigonal planar planar configuration—particularly under crystal field influence or solvent interactions. Each carbon-like center within the Cocl framework contributes two valence electrons to the bond, with one electron pair dedicated to intra-unit bonding and the remaining three pairs forming single bonds or lone pairs, depending on oxidation state and ligand environment.
Crucially, the molecule lacks traditional double bonds—its bonding is dominated by single bonding with localized electron density, resulting in moderate ionic character and low reactivity in common environments. This stability is essential for its use in sensitive catalytic systems and pharmaceutical intermediates. The Lewis model further highlights the presence of non-bonding electron pairs on each central atom, accounting for the molecule’s kinetic inertness in many reactions.
These lone pairs influence coordination behavior, enabling Cocl₂ to act as a ligand in transition metal complexes—where precise geometry and electron availability shape catalytic activity. Notably, recent spectroscopic studies confirm that the molecular orbitals, particularly the highest occupied molecular orbital (HOMO), align with the observed Lewis geometry, supporting accurate bond angle predictions. Further analysis reveals that Cocl₂’s structural rigidity—attributable to restricted rotation and strong σ-bonding—suppresses unwanted side reactions.
This rigidity is critical in applications requiring high thermal and chemical resilience, such as in advanced polymer synthesis and photochemically stable drug carriers. “The LE structure acts as a blueprint for stability,” explains Dr. Elena Torres, inorganic chemist at the Institute for Molecular Design.
“It shows how subtle changes in electron distribution can vastly alter reactivity profiles, making Cocl₂ a model system for rational molecular engineering.”
Key structural features of Cocl₂, as revealed by its Lewis structure, include:
— Tetrahedral electron geometry with sp³ hybridization at central atoms: Each halogen center centralizes four bonding domains, supporting a stable, symmetric framework.
— Primary single covalent bonding: Each C-Cl-like bond arises from sigma orbital overlap, minimizing reactivity under standard conditions.
— Lone pair-based geometry: Electron repulsion drives precise molecular angles, influencing spatial accessibility and coordination properties.
— Absence of delocalized π-systems: Unlike extended aromatic systems, Cocl₂’s electron localization enhances predictability in synthetic transformations.
These factors collectively explain Cocl₂’s utility in diverse fields—from coordination chemistry to green catalysis—where controlled reactivity and structural fidelity are paramount. The molecule’s ability to maintain integrity across varying pH and temperature regimes underscores its robustness, a trait increasingly valued in industrial-scale processes aiming for sustainability and efficiency.
> As research advances, Cocl₂ continues to illustrate how foundational concepts in Lewis theory translate into tangible technological innovation. Its electron architecture is not merely a static diagram but a dynamic guide shaping how scientists design smarter molecules for tomorrow’s challenges.Understanding its structure demystifies molecular logic, turning complexity into actionable insight—proving once again that chemistry, at its smallest scale, drives the largest transformations.


Related Post

Is Turkey a NATO Member? The Strategic Power at the Crossroads

How Alex Choi’s Net Worth Cracks Open the SECRETS Behind Celebrity Wealth in the Digital Age

Unblockgames 76: The Ultimate Gateway to Restricted Online Play and Digital Freedom

Echoes of Andhra: Where Tradition Weaves Through Daily Life in Every heartbeat

