BraceNeurons and AnchorTheNeuronsToCapillaries: Revolutionizing Brain Microcirculation Research
BraceNeurons and AnchorTheNeuronsToCapillaries: Revolutionizing Brain Microcirculation Research
At the frontier of neuroscience and microvascular biology lies a groundbreaking approach—brace neurons and anchor the neurons to capillaries—transforming how scientists visualize and interact with the brain’s most delicate networks. By stabilizing neuronal networks and tethering them precisely to the intricate capillary bed that sustains them, researchers are unlocking unprecedented insights into brain function, pathology, and potential therapies. This innovative technique enables real-time observation of neural activity at the capillary proximity, revealing dynamic interactions previously hidden within the brain’s labyrinthine vasculature.
Modern neuroscience has long sought to map neuronal connections within their physiological environment, yet isolating and stabilizing neurons while maintaining their natural spatial orientation near capillaries proved technically daunting. The breakthrough lies in a specialized framework—“brace neurons”—engineered to provide structural support without disrupting synaptic communication. Simultaneously, *AnchorTheNeuronsToCapillaries* employs bioadhesive scaffolds and micro-specific anchoring points that grip neuronal clusters at capillary junctions, ensuring both mechanical stability and biological fidelity.
“These anchoring systems preserve the microenvironment’s integrity while enabling high-resolution intraoperative and chronic imaging,” explains Dr. Elena Rojas, a neurovascular specialist at the Institute for Neural Dynamics. “By locking neurons in microdomains adjacent to capillaries, we capture neural responses not just electrically, but metabolically and hemodynamically.” The process begins with engineering neuron-anchoring constructs using biocompatible polymers that mimic extracellular matrix proteins.
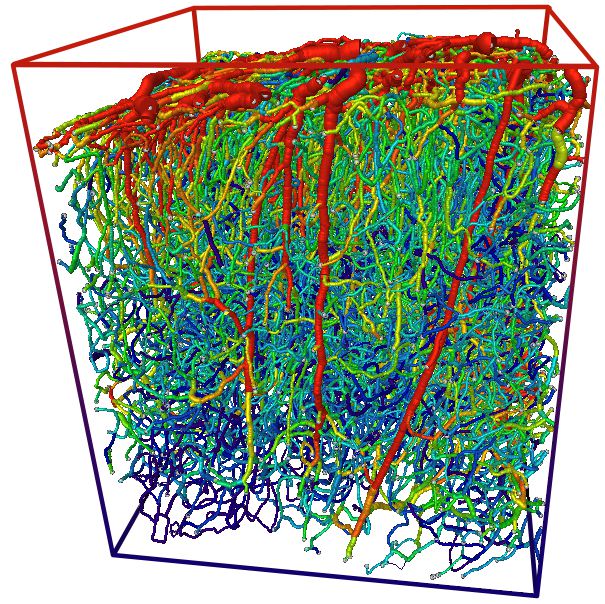
These materials promote selective adhesion to both neuron surfaces and capillary basement membranes, forming a resilient anchor network. Miniaturized titanium or silk-based tethers further stabilize position, allowing neurons to remain metabolically active while being imaged under high-magnification microscopy or functional MRI variants. This anchor strategy addresses a core limitation in neurovascular studies: maintaining functional proximity between neurons and capillaries, where oxygen, nutrients, and signaling molecules converge.
Conventional imaging often disturbs this delicate contact, yielding fragmented data. With neural elements firmly tethered near capillary networks, researchers now observe how localized blood flow fluctuations affect synaptic transmission and neuronal firing patterns in real time.
Anchoring neurons to capillaries reshapes experimental models, offering a new paradigm for studying neurodegenerative diseases, stroke recovery, and brain tumors.
In Alzheimer’s research, for instance, researchers can monitor amyloid-beta propagation in relation to vascular weakening with unprecedented precision. The technique also enhances optogenetic manipulation—by immobilizing targeted neuron clusters, scientists induce activity while observing capillary responses without motion artifacts.
From a technical standpoint, the integration of braced neurons with capillary anchoring relies on multi-scale precision. Microfabrication techniques produce scaffolds with micron-level architecture, tailored to match capillary geometries.Genetic labeling combined with super-resolution microscopy identifies optimal attachment sites on both neuronal dendrites and endothelial junctions. The anchoring process typically involves: - Selective neuron isolation using density gradient centrifugation - Surface modification with adhesive ligands that bind to integrins and vascular adhesion molecules - Deployment via minimally invasive microneedles to minimize tissue trauma - Real-time validation via fluorescent markers tracking neuron-vessel coupling
Despite its promise, the method faces challenges in long-term biocompatibility and immune response management. Chronic implants must resist glial scarring and endothelial degradation over months or years.
Moreover, precise spatial mapping remains computationally intensive, requiring AI-enhanced image registration to track thousands of braced neurons within the pulsatile capillary network. Technological convergence—nanomaterials, machine learning, and microfluidic delivery—now enables adaptive anchoring systems that adjust to real-time hemodynamic changes, maintaining neuron-capillary alignment even during blood pressure fluctuations.
Beyond research labs, the implications extend to regenerative medicine. Implantable neural interfaces anchored to host capillaries could restore circuit function in spinal cord injuries or Parkinson’s patients by stabilizing repaired networks at their metabolic heart.
Early clinical trials using biodegradable neuron scaffolds show encouraging results in restoring isolated sensory pathways.
The marriage of braced neurons and capillary anchoring represents more than a technical advance—it is a redefinition of how neurovascular systems are studied and understood. By tethering fragile neural circuits to their lifeline of capillaries, scientists gain a window into the brain’s true operational theater: a pulsing nexus where cognition, circulation, and pathology converge. As this technology matures, it promises not only deeper scientific insight but tangible pathways toward precision therapies for some of the most intractable neurological conditions.


Related Post

Who Owns Michelob Ultra? The Beer Behind the Hard-Hitting Health Narrative

The Mind-Bending World of JellyCollapseGame

Become A Learning Development Specialist: Your Path to Shaping Tomorrow’s Talent

Escape Reality in Animal Forest: The Disarming Power of Wildlife Simulation

